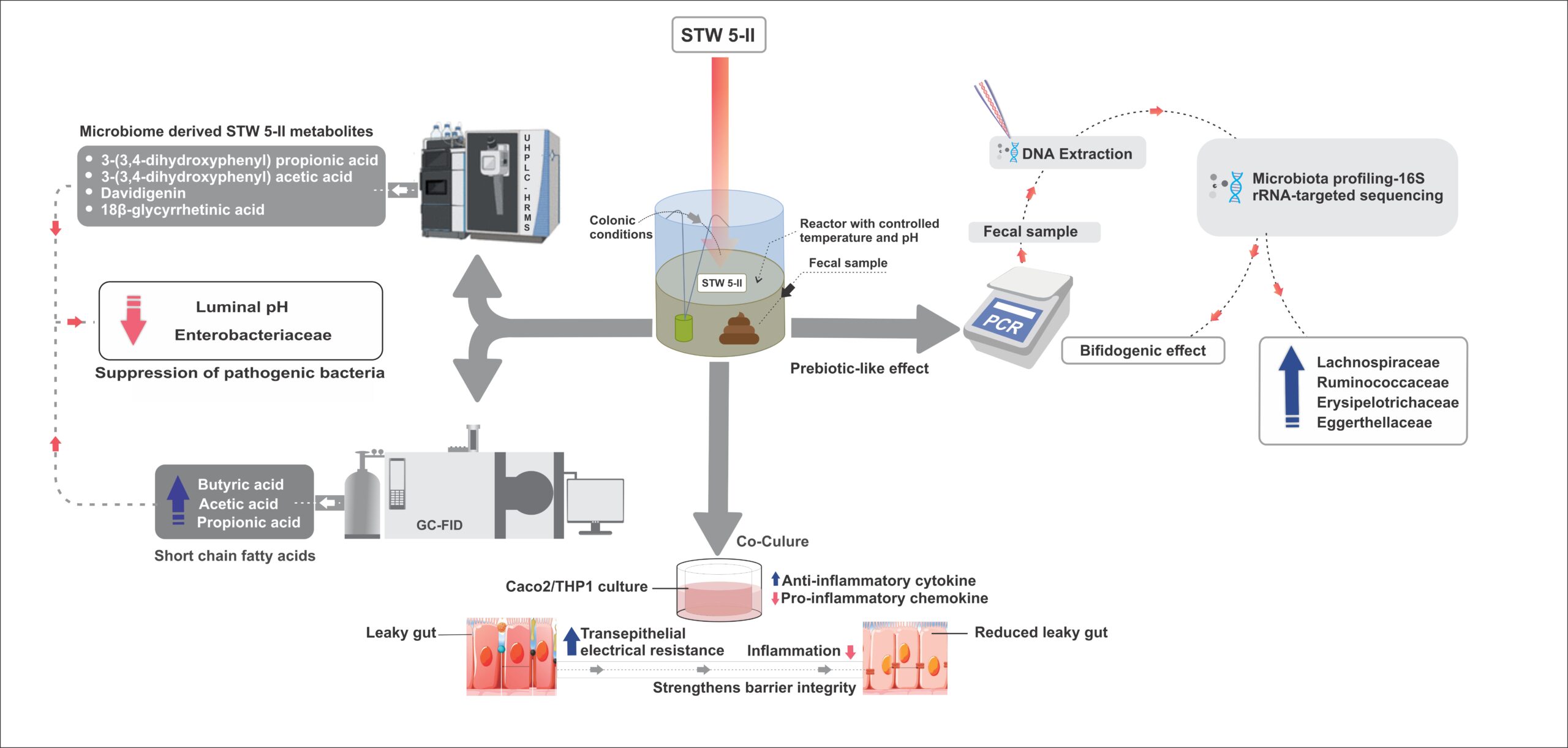
The evolving landscape of healthcare and pharmaceutical industries has catalysed a significant transformation in the traditional role of medical writers. Beyond content creation, medical writers now find themselves analysing and interpreting data, acting as strategic partners, and leveraging technology to deliver impactful and accurate medical content with data integrity.
Traditionally, medical writers were primarily responsible for simplifying complex scientific data into understandable formats. Their primary focus was communicating effectively with the target audience while maintaining accuracy, clarity, and compliance with industry guidelines. However, with significant advancements in the healthcare industry and increased industry demands, medical writers faced the challenge of efficiently managing and organising vast amounts of data while maintaining quality standards. This shift has required adopting advanced technologies to streamline workflows, ultimately enhancing efficiency.
Now, let’s delve deeper into Content Management Systems and examine Veeva Vault PromoMats as a prime example.
Understanding Content Management Systems (CMS)
Content Management Systems (CMS) are software applications that enable organizations to create, manage, and distribute digital content effectively. In the pharmaceutical and healthcare settings, where adherence to stringent regulations and standards is paramount, a robust CMS is essential, which would efficiently manage the extensive array of medical content, including promotional materials and regulatory documents. With the diversity of CMS platforms, organizations have options tailored to their needs. Veeva stands out as a leading CMS platform in pharma and healthcare settings. It offers robust features and capabilities designed to streamline content management processes.
Veeva Vault PromoMats: A Tailored Solution
Veeva Vault PromoMats is a cloud-based platform specifically designed for the healthcare sector. It stands out as a comprehensive solution tailored to meet the unique needs and challenges of managing promotional content. Veeva is a one-stop solution for creating, reviewing, approving, and distributing medical content. This manages the documents efficiently and guarantees version control and security of sensitive medical information.
Unveiling the Benefits of Veeva
- Centralized Content Repository: Medical writers can easily find approved content in one place, making sure all documents are consistent and accurate.
- Asset Organization and Search: With Veeva’s advanced search and sorting tools, finding promotional materials is quick and simple, boosting productivity.
- Integration: Veeva seamlessly fits into your workspace, enabling a single hub for managing and collaborating the content.
- Collaboration and Workflow Optimization: Medical writers can work closely with other teams, like researchers, medical experts, regulatory affairs, and marketing teams, using Veeva. Automation features speed up the review process, cutting down on waiting time.
- Content Reuse and Standardization: Veeva lets you reuse approved content across different documents, ensuring uniformity and meet regulations.
- Regulatory Compliance and Data Security: Veeva provides tools to stay compliant with regulations, keep track of changes, and securely store data, all within a safe cloud platform.
- Increased Efficiency and Productivity: Templates, content reuse, and dynamic formatting tools in Veeva make writing documents easier, so that writers can spend more time on content and less on layout/formatting.
Click Here:- Avoid These Common Pitfalls When Hiring Medical Writers
Transitioning to Veeva: Essential Requirements and Strategies
Platform Familiarization: Medical writers must extensively learn about the tool and its terminologies.
- Developing Proficiency: Formal training and hands-on practice are crucial for seamless tool integration in the workflow.
- Workflow Adjustments: Integrating Veeva may require adjustments to existing workflows, such as handling reviews, approvals, and content distribution.
- Team Collaboration and Communication: Adapting to new methods of collaboration, feedback, and revision tracking within the platform is essential for seamless teamwork.
- Staying Up to Date: Medical writers should stay informed about updates and adapt them in their workflows accordingly.
Incorporating Veeva into the Medical Writing Process: Overcoming Challenges
Incorporating Veeva into the medical writing process has its share of challenges, especially for healthcare professionals and medical writers. Healthcare professionals may face difficulties in syncing Veeva with existing systems and workflows within the healthcare organization while simultaneously using other tools and platforms. Similarly, medical writers rely on specific templates and formatting standards for their documents, making transitioning to a new platform like Veeva daunting due to its unique interface and complex features. Moreover, ensuring consistency and compliance of writing processes further adds to the complexity of the transition. Also, familiarization with the platform requires time and effort.
Overcoming Challenges with the Right Strategies and Support
However, with the right strategies and support, these challenges can be overcome, leading to a smoother integration and improved efficiency. Effective implementation of Veeva requires comprehensive support to align technology with medical writing expertise. Customized training initiatives play a crucial role in empowering healthcare professionals and medical writers to effectively utilize CMS platforms like Veeva to their fullest potential. These training programs should focus on providing user-friendly introduction to the platform’s unique features, allowing users to navigate Veeva with confidence and proficiency.
Conclusion
In the ever-evolving landscape of medical writing, the role of Content Management Systems (CMS) like Veeva Vault PromoMats is pivotal. As highlighted, these platforms streamline content creation, review, approval, and distribution processes, ensuring accuracy, compliance, and efficiency in the management of medical content. Transitioning to Veeva presents both opportunities and challenges for healthcare professionals and medical writers alike. While challenges such as platform familiarization, workflow adjustments, and integration complexities may arise, proactive strategies and comprehensive support can overcome these hurdles. By embracing customized training initiatives and leveraging technology-medical writing expertise alignment, organizations can empower their teams to effectively utilize CMS platforms like Veeva. Such initiatives enable healthcare professionals and medical writers to navigate the complexities of Veeva with confidence, maximizing its capabilities for improved efficiency and productivity in medical content management. In essence, by addressing challenges head-on and capitalizing on opportunities, the integration of Veeva into the medical writing process signifies a significant step forward in enhancing the quality, compliance, and effectiveness of medical content management in the healthcare industry.

Remember, the future of medical writing is not just about words – but to embrace technology for the betterment of mankind.
Turacoz is a registered content partner with Veeva, specializing in Veeva Vault PromoMats and other Veeva solutions. Our certification ensures expert guidance and support for seamless integration and effective utilization of Veeva platforms in medical content management.
Stay connected for more informative content on healthcare, technology, and professional development.










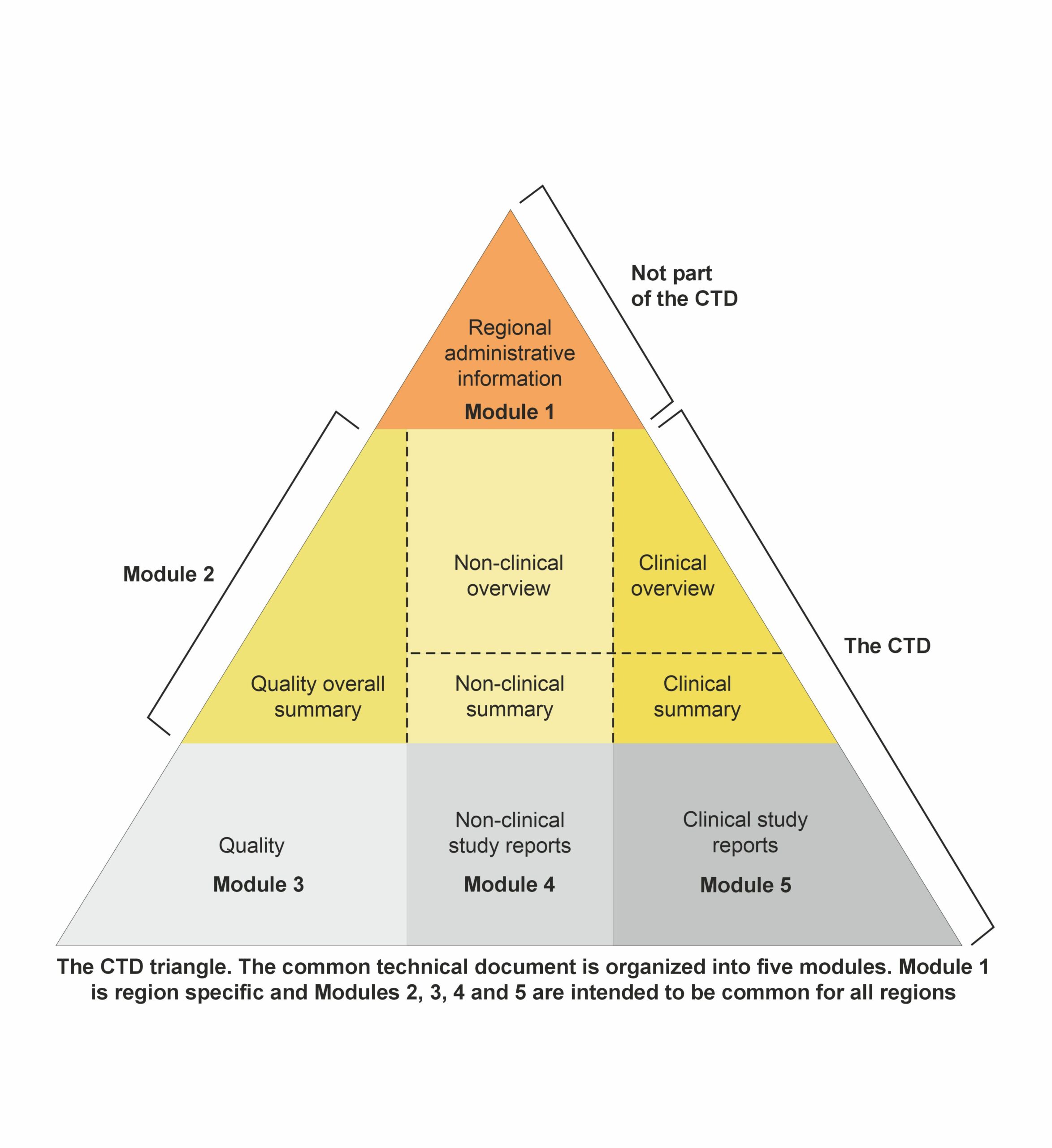 Module 1: Administrative and prescribing information
Module 1: Administrative and prescribing information