Real-world evidence (RWE) has emerged as a critical complement to randomized clinical trials (RCTs) in healthcare regulatory decision-making. While RCTs remain the gold standard to assess an intervention and the efficacy of an intervention and are irreplaceable when testing the effect of new treatments, they often face limitations in terms of generalizability, cost, and timeliness. Derived from real-world data (RWD), RWE offers valuable insights into the effectiveness, safety, and economic impact of healthcare interventions in everyday clinical settings. It has become an essential part of integrated evidence generation, creating a continuous flow of information from the premarket to post-market approval decisions. By providing a meaningful clinical context, RWE bridges the gap between evidence of efficacy and effectiveness, enriching the decision-making process.
Although RWE has been used for years to support post-marketing approval and drug safety, the 21st Century Cures Act, enacted in December 2016, accelerated the use of RWE for regulatory submission.1 The act, designed to speed up medical product development and deliver innovations to patients more efficiently, has led to an increase in the use of RWE.2 For example, between 2017 and 2019, only 13% of evaluated oncology submissions to the FDA included RWE to support efficacy, compared to 70% from 2019 to 2021 using RWE to support efficacy and/or safety.1
The Role of Real-World Evidence in Regulatory Decision-Making
- Complementing RCTs
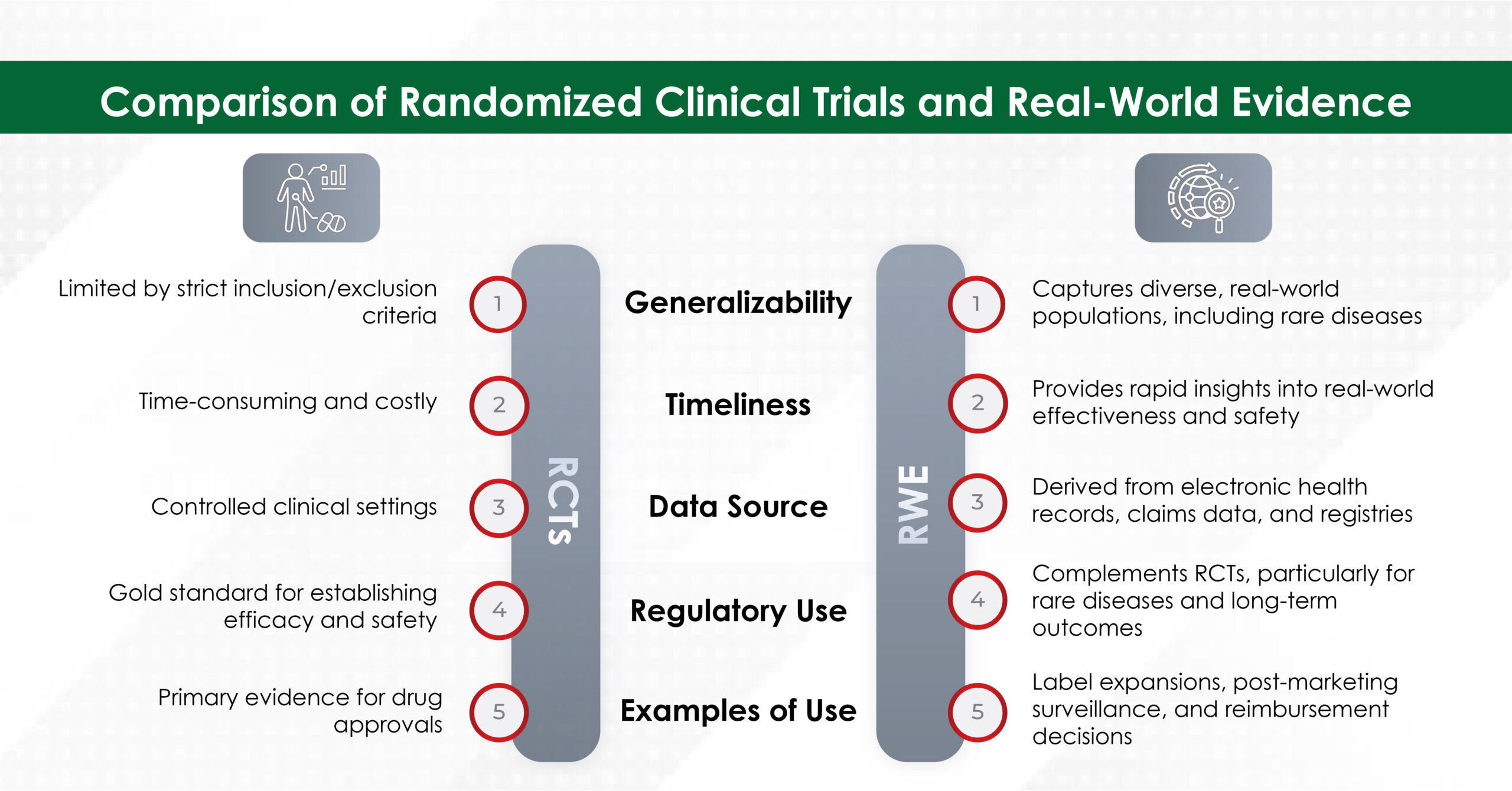
RCTs are highly controlled studies that provide robust evidence of efficacy and safety but are often limited by their strict inclusion and exclusion criteria, which may not reflect real-world patient populations. On the other hand, RWE captures data from diverse real-world settings, including patients with comorbidities, rare diseases, and those taking multiple medications, thereby enhancing the generalizability of the findings. Additionally, the RWE allows for the assessment of intervention values, considering both clinical and economic implications. Understanding real-world costs and outcomes is essential for optimizing resource allocation and ensuring the greatest value to patients and society.3,4
- Addressing Evidence Gaps
RWE is particularly valuable in addressing questions that RCTs cannot answer or do not answer. For example, RWE can provide long-term safety and effectiveness data, information on drug interactions, assess treatment outcomes in rare diseases, and evaluate the impact of medications in paediatric or geriatric populations.5,6 Additionally, RWE can inform decisions on drug labelling, post-marketing surveillance, and reimbursement.7
- Regulatory Applications
Regulatory bodies such as the U.S. Food and Drug Administration (USFDA), European Medicines Agency (EMA), and Health Canada have increasingly recognized the value of RWE in regulatory decision-making. In North America, both the FDA and Health Canada have created frameworks to integrate RWE into the regulatory process for premarket and post-market activities. The FDA’s 2018 framework emphasizes using RWE to modify drug labels and evaluate effectiveness, whereas Health Canada encourages its use for special populations and rare diseases. In Europe, the EMA has set out strategies, including the “Regulatory Science to 2025” document and Adaptive Pathways, to incorporate RWE throughout the drug lifecycle, with a focus on harmonizing data access and analysis within the EU. In Asia, countries such as China, Japan, and Taiwan, have developed specific guidelines for the use of RWE in drug development. China’s National Medical Products Administration (NMPA) emphasizes its use for rare diseases and label expansions, while Japan’s Pharmaceuticals and Medical Devices Agency (PMDA) is working to refine methods for ensuring the reliability of registry data.8
Examples of RWE Improving Decision-Making
- COVID-19 Vaccine Deployment
During the COVID-19 pandemic, RWE was crucial in speeding up vaccine deployment by assessing vaccine effectiveness (VE) in real-world settings and complementing clinical trial data. Studies conducted in Denmark and Israel have provided valuable insights into VE against emerging variants and the effectiveness of booster doses. RWE also helped refine vaccination strategies and dosing regimens, offering evidence that RCTs couldn’t deliver on time. It supported safety monitoring and vaccine adaptation to new variants, enabling regulatory bodies to adjust dosing, guide on target populations, and optimize vaccine strategies for rapid decision-making.9
- Rare Disease Medicine Approvals
In the context of rare diseases, the RWE has been instrumental in broadening the indicated population for orphan drugs. A study analysing engagements with the EMA found that submissions, including RWE, resulted in a broader indicated population than those relying solely on RCT evidence. This highlights the potential of RWE to expand treatment access for rare diseases.10
- Medical Cannabis
RWE has also been used to support the approval of medical cannabis, where RCT evidence is often limited due to the challenges of studying whole-plant medicines. RWE studies, incorporating patient-reported outcomes, have demonstrated the positive impact of medical cannabis on patients’ lives, providing a broader evidence base for regulatory decisions.11
Challenges and Limitations of RWE
While RWE offers significant advantages, it also has limitations. Key challenges include concerns about data quality, potential biases, and lack of standardization in data collection and analysis. Additionally, RWE studies may lack the rigor of RCTs, and their findings may be influenced by confounding factors.4,6 To address these challenges, regulatory bodies stress the need for fit-for-purpose data, ensuring transparent study designs, and applying robust analytical methods.12
The Future of RWE in Regulatory Decision-Making
The integration of RWE into regulatory decision-making is expected to grow as methodologies improve and data quality is enhanced. Emerging trends, such as the use of artificial intelligence in healthcare and patient-generated data, offer new opportunities for leveraging RWE.4,13 Additionally, hybrid trial designs that combine elements of RCTs and RWE are being explored to generate evidence that is both robust and generalizable.14
Conclusion
Real-world evidence has become an indispensable tool for healthcare regulatory bodies, complementing the strengths of RCTs and addressing their limitations. By providing insights into real-world effectiveness, safety, and health economics impact, RWE has improved decision-making in areas where RCT data is insufficient. As methodologies evolve and data quality improves, the role of RWE in regulatory decision-making is expected to expand, offering new opportunities for advancing public health.
Turacoz specializes in leveraging RWE to support regulatory submissions, market access strategies, and healthcare decision-making. With expertise in data analytics, evidence synthesis, and regulatory compliance, we help pharmaceutical, and healthcare organizations integrate RWE into their product development and approval processes. From designing fit-for-purpose RWE studies to ensuring alignment with global regulatory frameworks, our tailored solutions empower clients to generate high-quality, actionable insights. By partnering with us, companies can enhance their evidence-generation strategies, optimize resource allocation, and accelerate patient access to innovative treatments.
References:
- Wilson BE, Booth CM. Real-world data: bridging the gap between clinical trials and practice. EClinicalMedicine. 2024;78:102915.
- Real-World Evidence. Available at: https://www.fda.gov/science-research/science-and-research-special-topics/real-world-evidence#:~:text=The%2021st%20Century%20Cures%20Act,drug%20post%2Dapproval%20study%20requirements. Last accessed: March 2025.
- Hernandez RK, Critchlow CW, Dreyer N, et al. Advancing Principled Pharmacoepidemiologic Research to Support Regulatory and Healthcare Decision Making: The Era of Real-World Evidence. Clin Pharmacol Ther. Published online January 14, 2025.
- Bhatia N. Harnessing real-world evidence in pharmacoeconomics: A comprehensive review. Open Health. 2024:5(1).
- Prilla S, Groeneveld S, Pacurariu A, et al. Real-World Evidence to Support EU Regulatory Decision Making-Results From a Pilot of Regulatory Use Cases. Clin Pharmacol Ther. 2024;116(5):1188-1197.
- Bakker E, Plueschke K, Jonker CJ, et al. Contribution of Real-World Evidence in European Medicines Agency’s Regulatory Decision Making. Clin Pharmacol Ther. 2023;113(1):135-151.
- Pulini AA, Caetano GM, Clautiaux H, et al. Impact of Real-World Data on Market Authorization, Reimbursement Decision & Price Negotiation. Ther Innov Regul Sci. 2021;55(1):228-238.
- Burns L, Roux NL, Kalesnik-Orszulak R, et al. Real-World Evidence for Regulatory Decision-Making: Guidance From Around the World. Clin Ther. 2022;44(3):420-437.
- Bollaerts K, Wyndham-Thomas C, Miller E, et al. The role of real-world evidence for regulatory and public health decision-making for Accelerated Vaccine Deployment- a meeting report. Biologicals. 2024;85:101750.
- Jandhyala R. The effect of adding real-world evidence to regulatory submissions on the breadth of population indicated for rare disease medicine treatment by the European Medicines Agency. Pharm. Policy Pract. 2022:15.
- Schlag AK, Zafar RR, Lynskey MT, et al. The value of real world evidence: The case of medical cannabis. Front Psychiatry. 2022;13:1027159.
- Dreyer NA, Hall M, Christian JB. Modernizing Regulatory Evidence with Trials and Real-World Studies. Ther Innov Regul Sci. 2020;54(5):1112-1115.
- Dagenais S, Russo L, Madsen A, et al. Use of Real-World Evidence to Drive Drug Development Strategy and Inform Clinical Trial Design. Clin Pharmacol Ther. 2022;111(1):77-89.
- Andre EB, Reynolds R, Caubel P, et al. Trial designs using real-world data: The changing landscape of the regulatory approval process. Pharmacoepidemiol Drug Saf. 2020;29(10):1201-1212.