Regulatory medical writing is a specialized field that involves developing and managing documentation necessary to obtain regulatory approval for medical products such as pharmaceuticals, biologics, and medical devices. It involves drafting various medical documents such as clinical trial protocols, clinical trial reports, regulatory submissions, investigator brochures, patient information leaflets, and pharmacovigilance reports. Regulatory compliance is crucial in healthcare and pharmaceuticals. It ensures patient safety through reliable information. Let’s explore regulatory medical writing, its compliance, the challenges associated with maintaining regulatory compliance, the qualities of regulatory medical writers, and the latest trends in compliance standards.
Understanding Regulatory Requirements
Regulatory requirements are essential in medical writing. Understanding these criteria is crucial to ensuring compliance in the healthcare industry. Regulatory authorities such as the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have established various guidelines for creating medical documents. These guidelines include precise formatting guidelines, clinical trial and research documentation, and compliance with ethical standards. Adhering to these regulatory requirements and industry norms enables you to communicate critical information effectively.
Importance of Regulatory Compliance
The regulatory documentation must be comprehensive and precise, adhering to strict regulations and guidelines to ensure that healthcare professionals receive reliable, evidence-based information. Compliance is crucial to maintain the highest standards of patient safety. Failure to comply with these regulations can cause delays in the drug approval process and, in some cases, lead to severe consequences, including legal issues, product recalls, and patient harm. Therefore, adherence to regulations is of utmost importance in medical writing to ensure patient safety and practitioner access to credible information.
You must strictly adhere to regulatory requirements while crafting the documents for an efficient drug development process. The documents that need to be compliant with regulatory requirements include clinical study reports (CSRs), clinical study protocols (CSPs), investigational new drug applications (INDAs), new drug applications (NDAs), and marketing authorization applications (MAAs).
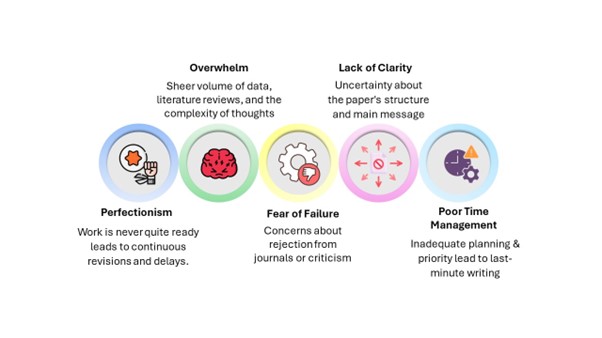
Challenges in Maintaining Regulatory Compliance
The field of medical writing is constantly evolving and poses several challenges for you to be compliant. Some common issues that arise include:
Keeping up with changing regulations: You must stay updated with the latest guidelines and adapt your writing to the evolving regulatory requirements.
Scientific complexity: Medical writing often involves highly complex scientific concepts and terminology. In some situations, it can be difficult to ensure accuracy and clarity while maintaining compliance.
Balancing transparency and confidentiality: In medical writing, you provide transparent information while protecting patient confidentiality. This can be particularly challenging when writing about sensitive topics or clinical trial results.
Collaboration and coordination: Medical writing often involves coordination with multiple stakeholders, including researchers, clinicians, and regulatory authorities. Coordinating inputs from different sources while maintaining compliance may be challenging.
Qualities of Regulatory Medical Writers
Regulatory medical writers are instrumental in creating documents that comply with regulatory standards and aid in the timely approval and accessibility of life-saving medications and therapies in the market.
You must have specific key characteristics to become a regulatory medical writer:
- A thorough understanding of global regulatory guidelines ensures the drug development program complies with all relevant regulations.
- Strong communication skills are necessary for effective engagement with cross-functional teams in clinical research, medical affairs, and regulatory affairs. This is critical for ensuring all stakeholders are informed and aligned throughout the drug development process.
- Regulatory writers must be able to translate complex clinical data into clear and concise language for regulatory submissions and other documents.
Click Here:- Mastering Regulatory Writing Skills Online
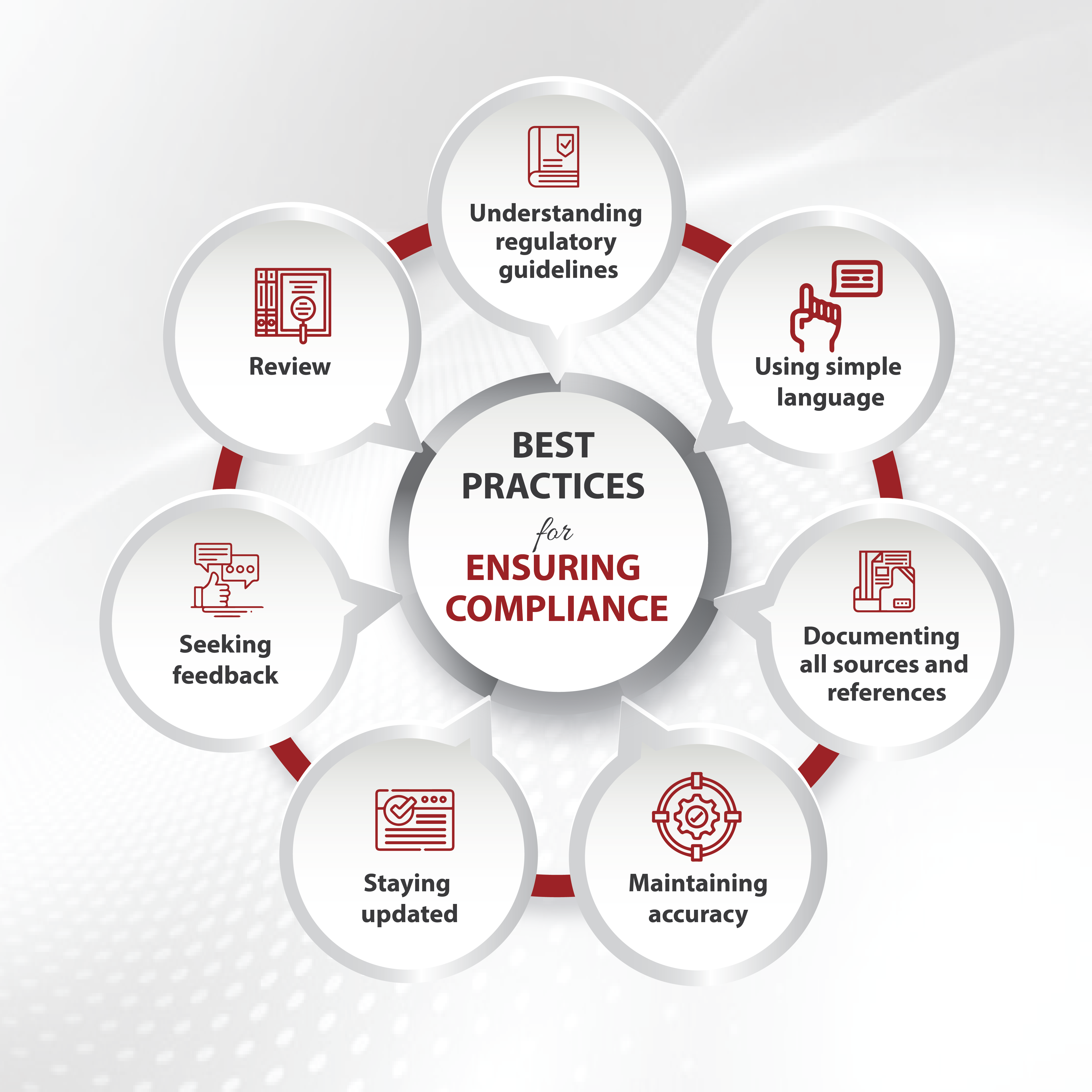
Best Practices for Ensuring Compliance
Adhering to best practices can improve the quality and regulatory compliance of your writing, ultimately contributing to healthcare’s overall safety and effectiveness. This involves:
Understanding regulatory guidelines: Stay updated with regulations and guidelines from regulatory bodies like the FDA and EMA and familiarize yourself with requirements for medical documents.
Using simple language: Always use clear, concise, and easily understandable language for the target audience. Avoid jargon and technical terms whenever possible.
Maintaining accuracy and staying updated: Always provide accurate and up-to-date information supported by scientific evidence.
Seeking feedback and review: Collaborate with peers and subject matter experts to review and provide feedback on the generated medical content. It helps ensure accuracy and compliance.
Documenting all sources and references: Properly document all sources and references used in medical writing to ensure transparency and avoid plagiarism.
Trends in Compliance Standards
Compliance standards should be evolved in medical writing to keep up with technological advancements, regulatory requirements, and the increasing need for transparency and patient-centricity. Several emerging trends are shaping the future of compliance standards in medical writing. These include:
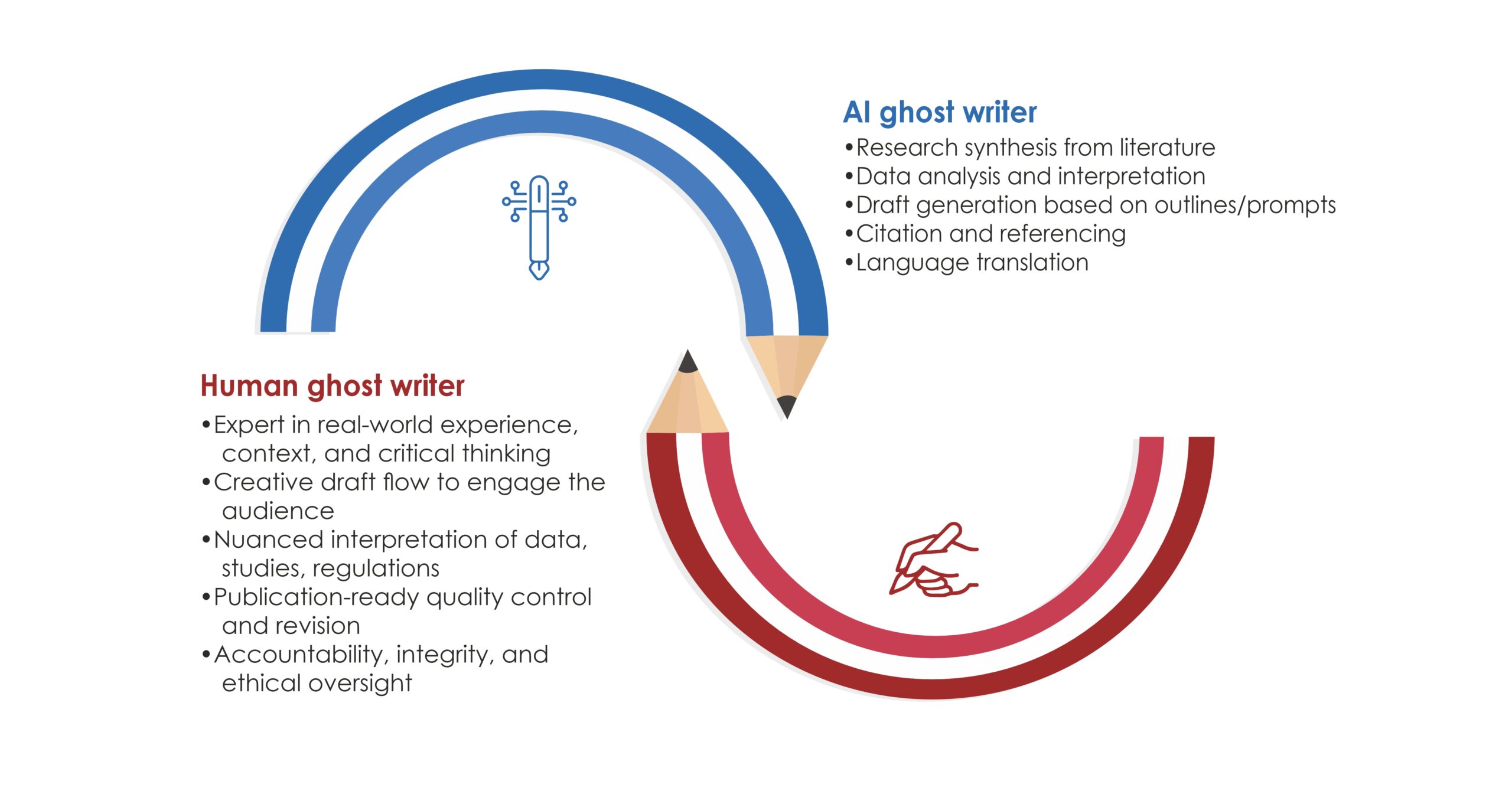
Use of artificial intelligence (AI): AI technologies have the potential to automate certain aspects of medical writing, like data analysis and report generation. However, ensuring compliance with regulatory guidelines will remain critical to AI-driven medical writing.
Integration of real-world evidence: Real-world evidence derived from sources like electronic health records and patient registries is becoming more important in medical research. Compliance standards must address the unique challenges and opportunities of incorporating real-world evidence into medical writing.
Focus on patient engagement and communication: Patient-centricity is gaining significance in healthcare. Compliance standards will likely emphasize the need for clear and patient-friendly communication in medical writing, ensuring patients can understand and make informed decisions about their healthcare.
Enhanced data privacy and security: As concerns over data privacy grow, compliance standards will continue to evolve to protect patient information and adhere to data privacy regulations.
Staying updated with these future trends allows you to proactively adapt to practices and maintain compliance in an ever-changing regulatory landscape. Regulatory writing is crucial for pharmaceutical success, including compliance, clinical development, approval, marketing, and branding. It’s becoming even more critical with evolving requirements.
Are you interested in becoming a skilled regulatory medical writer? Grab this opportunity to join Turacoz! We are a committed global organization that makes healthcare information accessible through empowering training programs with our professionals, who will help you achieve your goal with confidence. Join our community of writers and take the first step towards a successful career. Please email us at [email protected] to learn more and get started today!