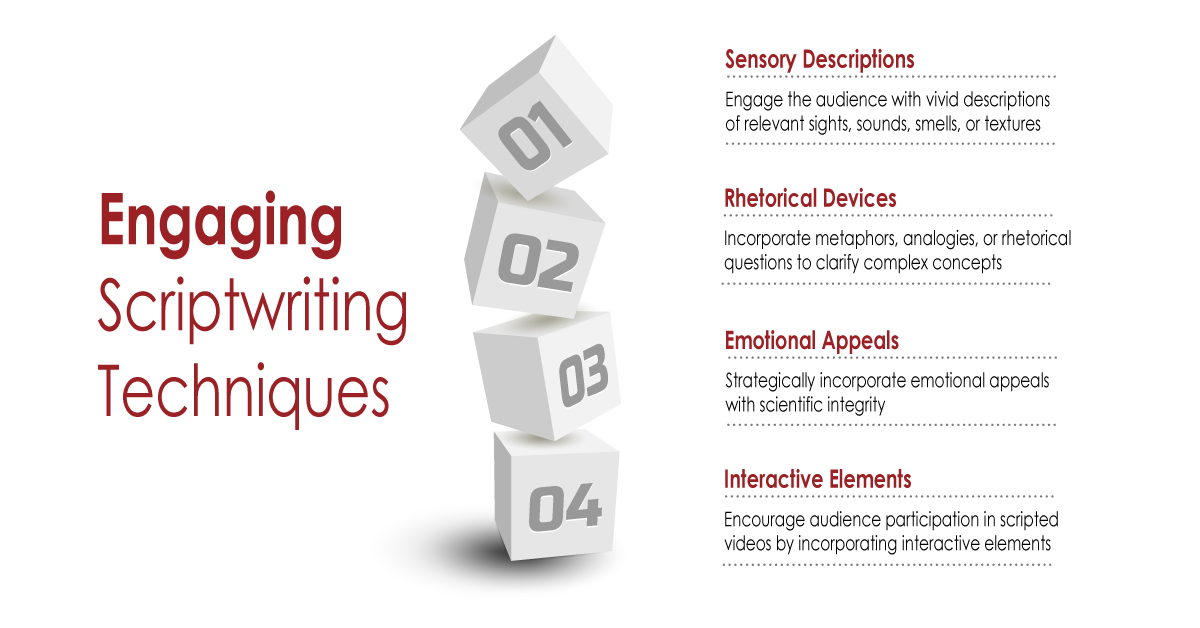
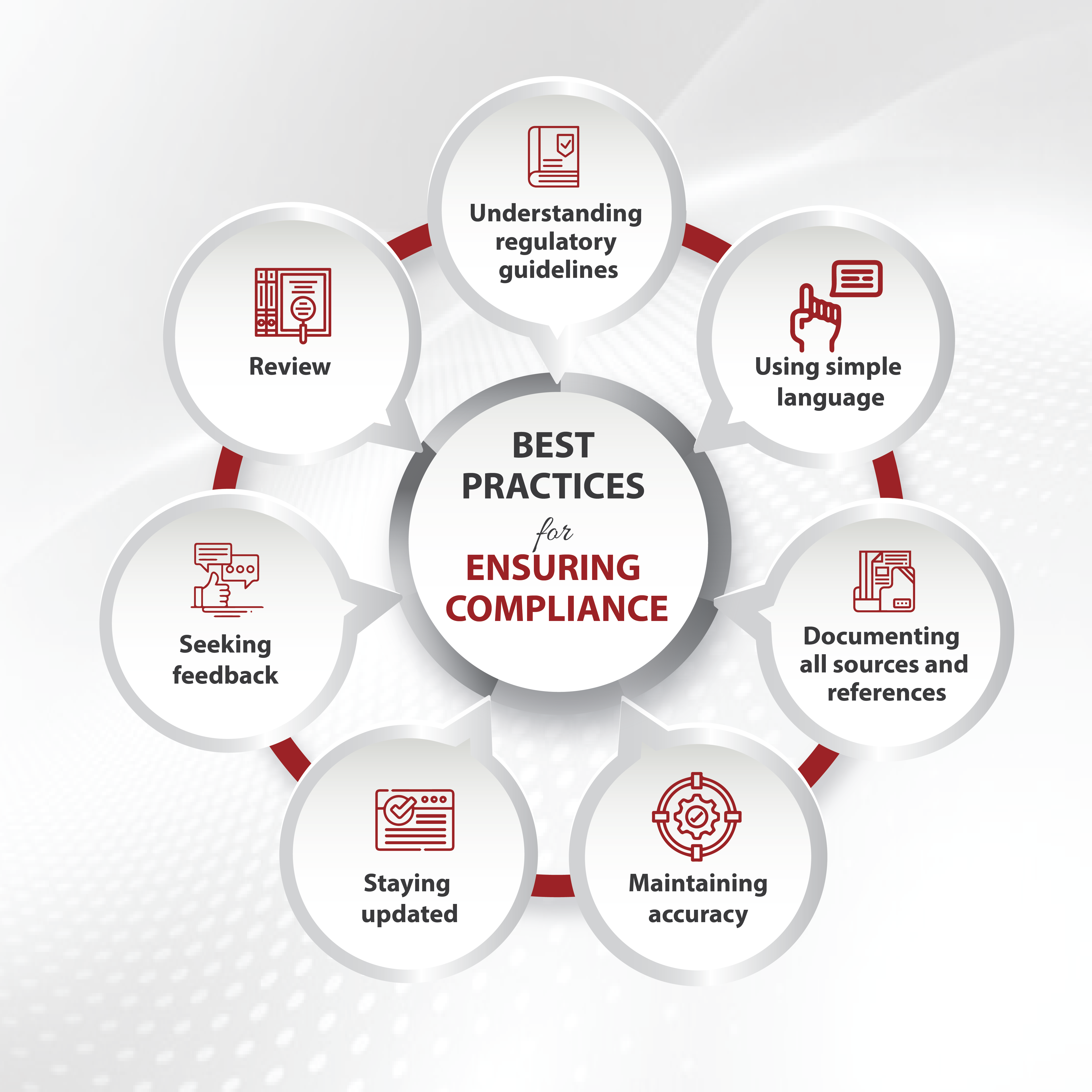
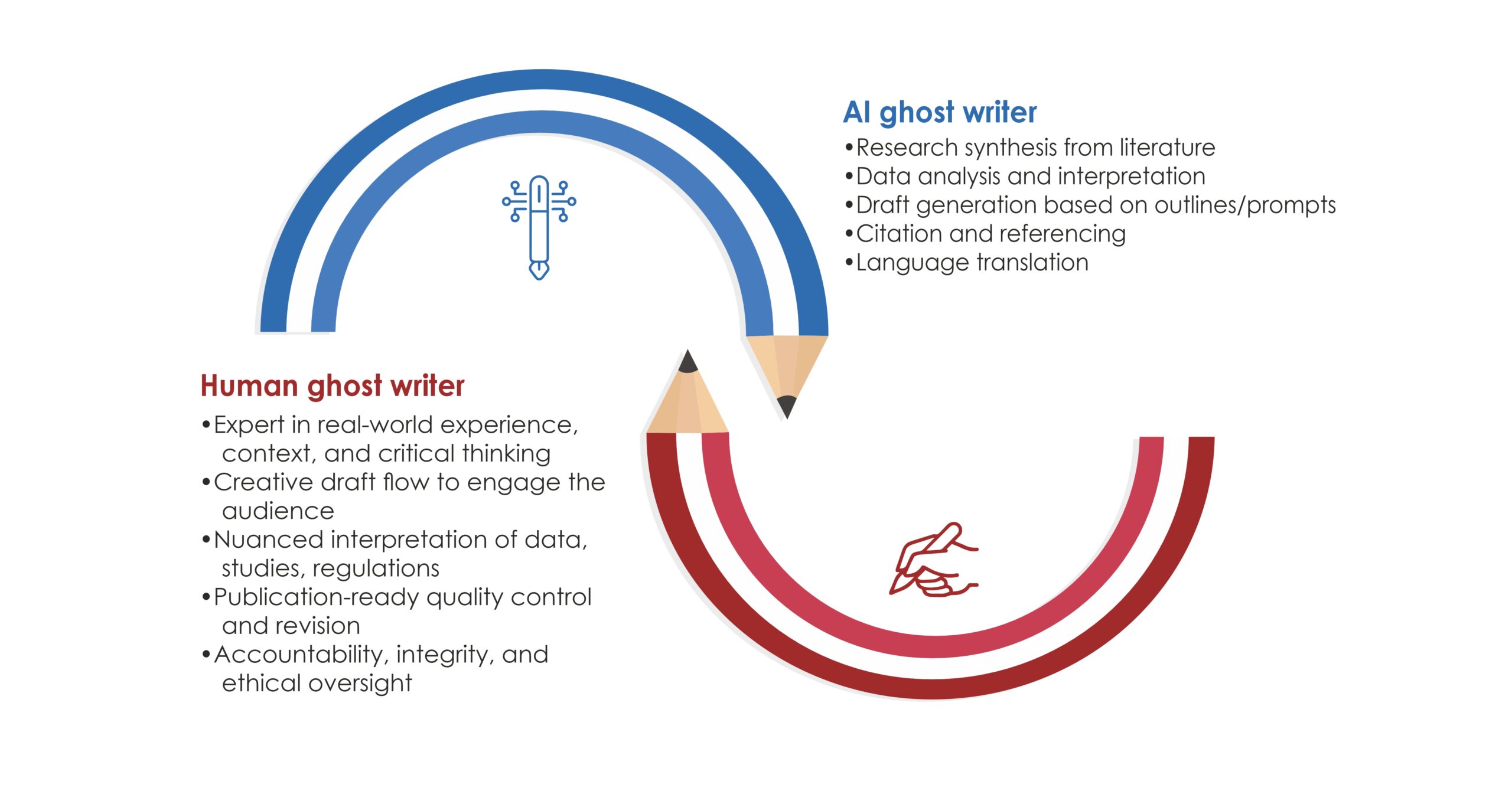
In the rapidly evolving landscape of healthcare and scientific research, effective communication is crucial. Medical writing, which encompasses a broad range of documents from clinical trial reports to patient education materials, is undergoing a significant transformation thanks to advancements in technology. Among these advancements, Canva and various AI-driven tools are revolutionizing how medical information is presented, written, and disseminated. This blog explores the impact of Canva and other AI tools in medical writing, highlighting their contributions, challenges, and future potential.
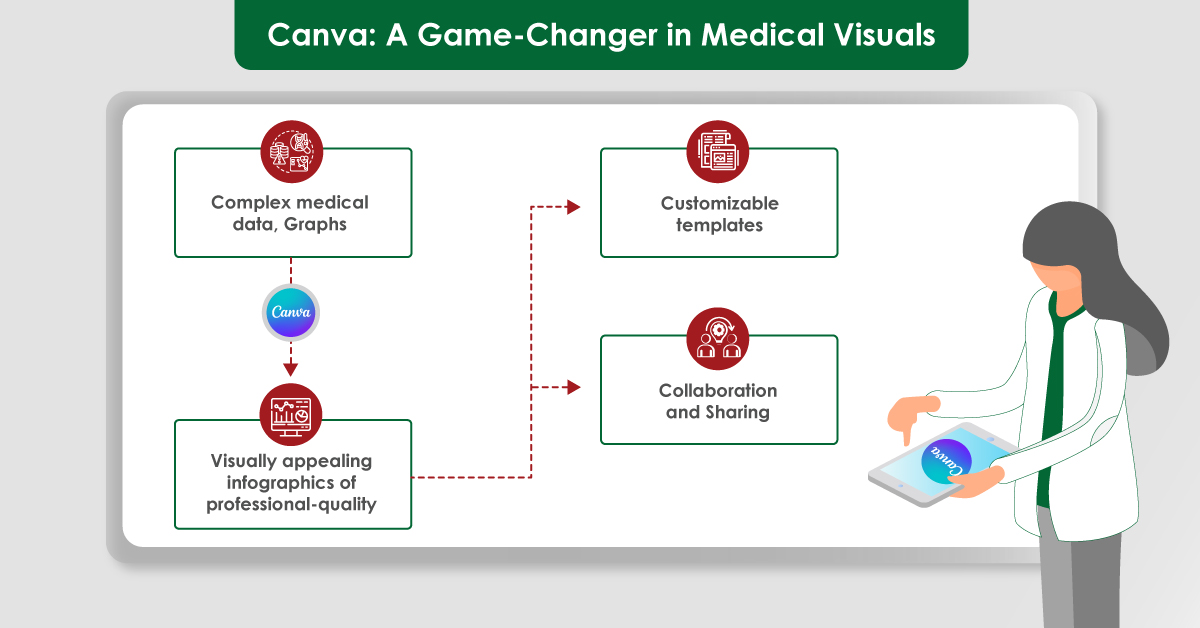
Canva: A Game-Changer in Medical Visuals
- Enhancing Presentation of Medical Data: A go-to tool for creating visually appealing graphics, making complex medical data more accessible and understandable. Its user-friendly interface allows medical writers to design professional-quality charts, graphs, and infographics without advanced design skills.
- Customizable Templates for Consistency: Its extensive library of customizable templates helps maintain consistency across various documents, whether creating patient education brochures, research posters, or clinical reports. Consistent design looks professional but helps reinforce brand identity and ensures key messages are communicated effectively.
- Collaboration and Sharing: This feature enables multiple users to work on a project simultaneously, which is useful for team-based medical writing projects. Large research teams or departments can collaborate in real-time, ensuring that all members are on the same page. Furthermore, Canva’s sharing options allow for easy distribution of completed designs, whether through direct links, social media, or embedding in websites.
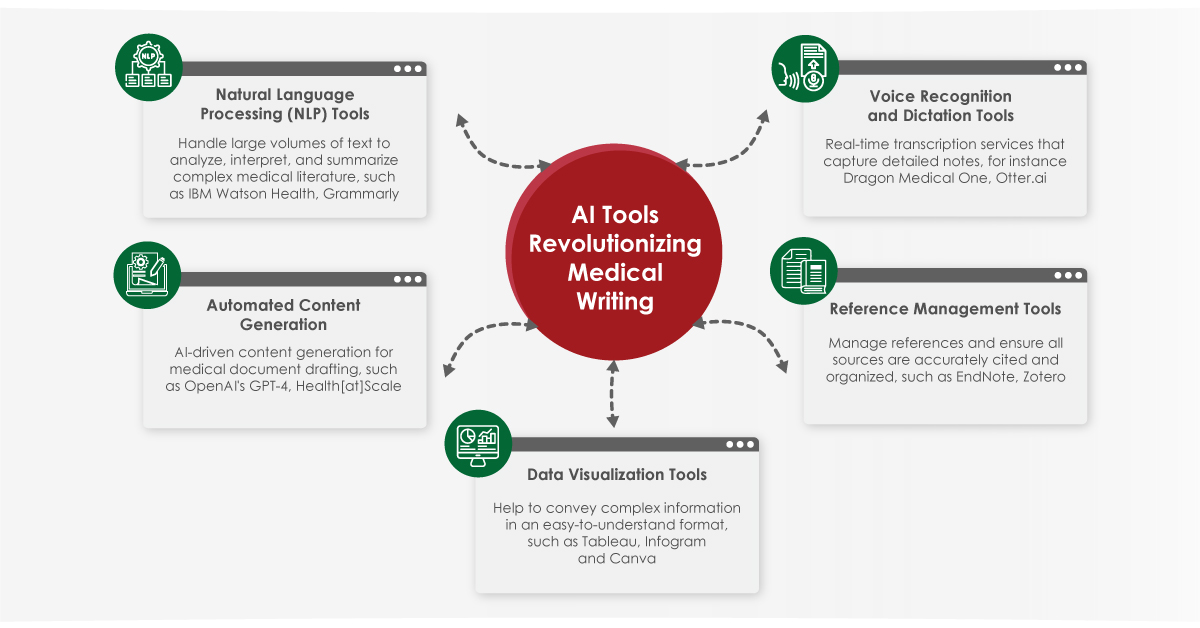
Other AI Tools Natural Language Processing (NLP) Tools: NLP tools have revolutionized how medical writers handle large volumes of text, offering capabilities to analyze, interpret, and summarize complex medical literature.
- IBM Watson Health: This tool uses NLP to scan and analyze vast amounts of medical literature, clinical trial data, and patient records. IBM Watson Health can identify patterns and generate hypotheses, assisting medical writers in creating evidence-based content. It provides comprehensive reviews of current research, facilitating the creation of up-to-date and accurate medical documents.
- Grammarly: Grammarly is another NLP tool invaluable for medical writers. It offers advanced grammar and style checking, ensuring documents are clear, concise, and error-free. Its ability to suggest synonyms and restructure sentences enhances the readability and professionalism of medical writing.
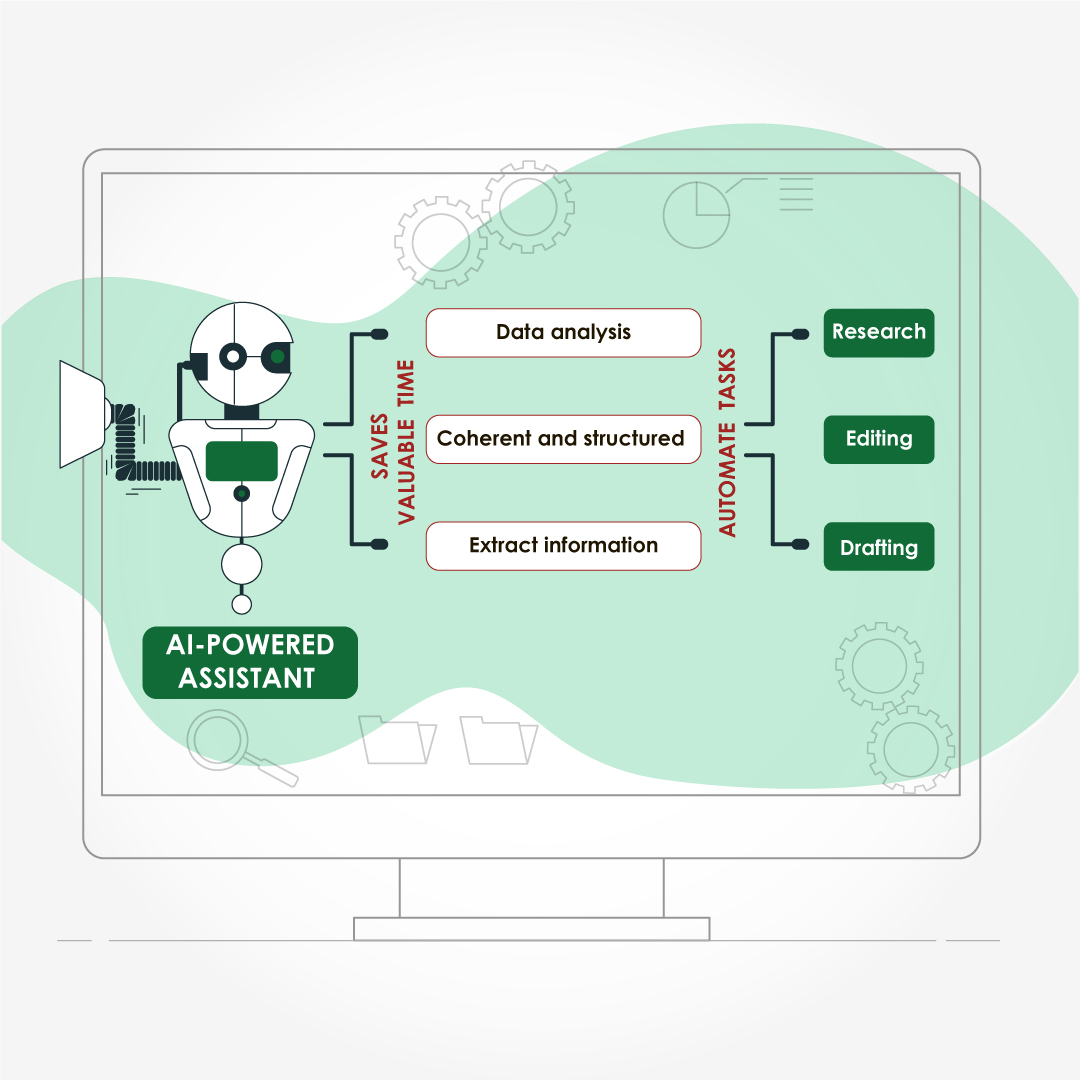
Automated Content Generation
AI-driven content generation tools assist in drafting medical documents, saving time and ensuring consistency.
- OpenAI’s GPT-4: It can produce coherent and contextually relevant text based on input data, making it useful for drafting research articles, patient education materials, and clinical documentation. While human oversight is necessary to ensure accuracy, GPT-4 significantly reduces the initial drafting workload.
- Health[at]Scale: This tool uses machine learning to predict health outcomes and personalize treatment recommendations. It generates patient-specific reports that summarize health risks and suggest preventive measures, which is invaluable in creating personalized patient education materials and clinical summaries.
Data Visualization Tools
Effective data visualization is crucial in medical writing, helping convey complex information in an easily digestible format.
- Tableau: A powerful data visualization tool that enables medical writers to create interactive and detailed charts, graphs, and dashboards. Particularly, useful for presenting research data, epidemiological trends, and clinical trial results. Its ability to handle large datasets and perform real-time updates makes it indispensable for dynamic data presentation.
- Infogram: A user-friendly platform for creating engaging infographics using templates and design elements. In medical writing, infographics can simplify the presentation of statistics, treatment protocols, and patient care guidelines.
Read More:- How Can You Benefit from Medical Communication services?
Voice Recognition and Dictation Tools
Voice recognition and dictation tools are transforming how medical writers compose and edit documents, making the process faster and more efficient.
- Dragon Medical One: One is a leading voice recognition software designed specifically for healthcare. It allows medical professionals to dictate clinical notes, patient reports, and other documents with high accuracy, speeding up the documentation process and reducing the physical strain of typing.
- ai: A real-time transcription service, that captures detailed notes during meetings, conferences, and interviews.
Reference Management Tools
A critical aspect of medical writing is managing references, and ensuring all sources are accurately cited and organized.
- EndNote: A comprehensive reference management tool helps to organize and format citations and bibliographies. It integrates with word processing software to streamline the citation process, reducing the likelihood of errors and ensuring adherence to various citation styles.
- Zotero: An open-source reference management tool allows users to collect, organize, and cite research sources. Its browser extension captures citation information directly from web pages, while its collaborative features support teamwork in large research projects.
Challenges and Considerations
While AI tools offer numerous advantages, their integration into medical writing presents challenges and considerations.
- Ensuring Accuracy: AI-generated content must be carefully reviewed for accuracy. Although AI tools can process and generate text based on vast datasets, they may not fully grasp the nuanced context of medical information. Human oversight is essential to verify the correctness of the content and make necessary adjustments.
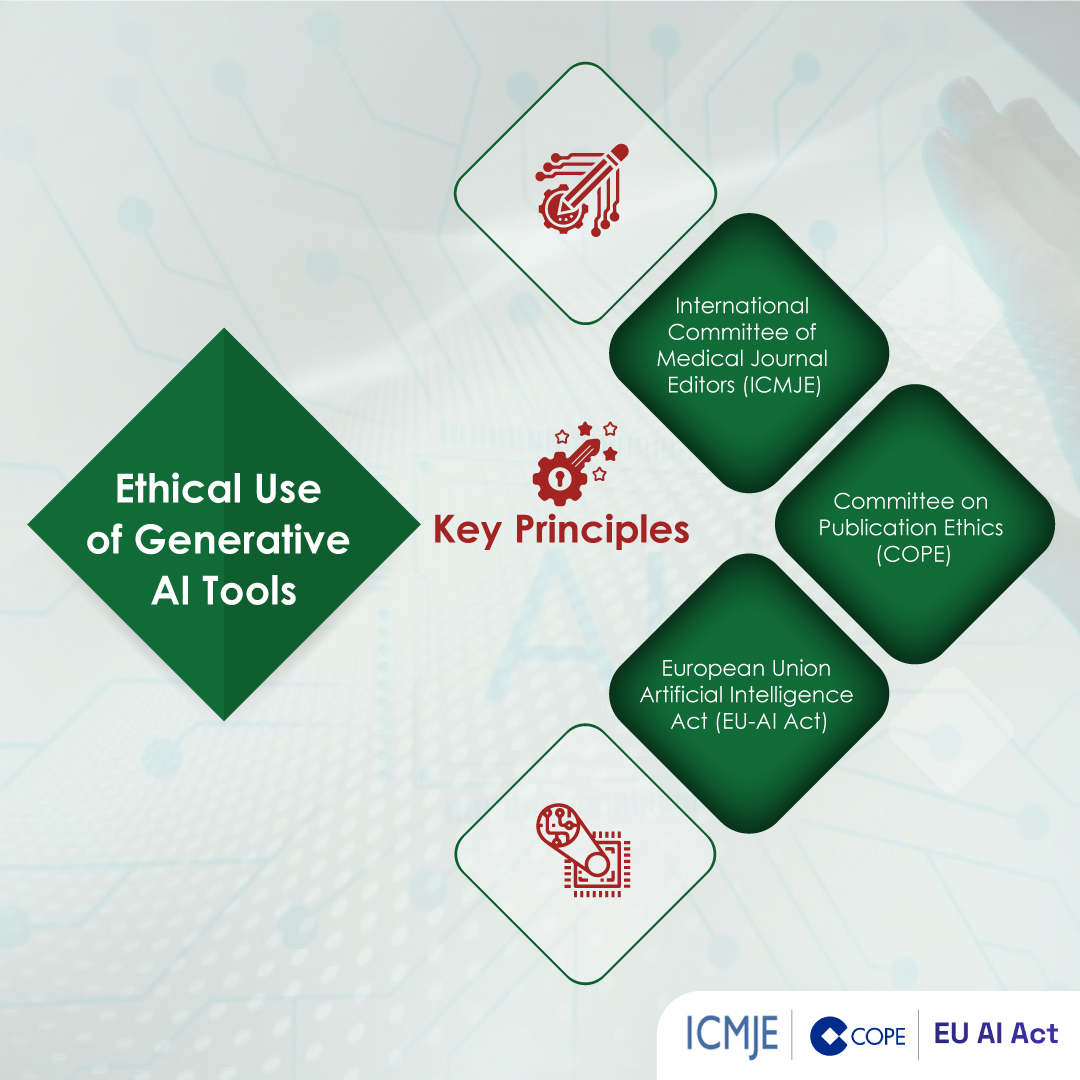
- Ethical Concerns: Transparency and accountability-related concerns arise due to use of AI in medical writing. Medical writers should disclose the use of AI tools in their work to maintain trust and integrity. Additionally, guidelines on the ethical use of patient data and AI-generated content must be established.
- Data Privacy: AI tools must comply with regulations to protect patient information. Ensuring that AI tools are secure, and that data is anonymized where necessary is crucial in maintaining confidentiality.
- Adaptation and Training: Adopting new AI tools requires training and adaptation. Medical writers need to familiarize themselves with the functionalities and limitations of these tools to use them effectively. Continuous learning and staying updated with technological advancements are essential for maximizing the benefits of AI.
Conclusion
The integration of Canva and other AI tools is transforming the field of medical writing, making it more efficient, accurate, and accessible. These technologies offer powerful capabilities for data visualization, automated content generation, reference management, and more, enhancing the way medical information is communicated. However, the successful adoption of these tools requires careful consideration of accuracy, ethics, data privacy, and ongoing training.
As AI continues to evolve, its potential to revolutionize medical writing will only grow, promising a future where medical communication is more effective and impactful. We at Turacoz, embrace these advancements and address the associated challenges, our medical writers can leverage technology to enhance their work, ultimately contributing to better healthcare outcomes and scientific progress.
Interested in taking the first steps into medical writing or enhancing your expertise to meet the challenges of omnichannel communication in healthcare, we invite you to explore the opportunities available through our training programs. Contact us at https://turacoz.com/ to learn how you can join the ranks of medical writers making a significant impact in healthcare communication. Together, let’s shape a future where accurate, accessible, and actionable health information reaches every corner of the globe, empowering individuals and transforming healthcare outcomes.