In the rapidly evolving field of medical research, the application of artificial intelligence (AI) has brought transformative changes. Among the various AI-driven technologies, predictive analytics has emerged as a crucial tool. By leveraging historical data, statistical algorithms, and machine learning techniques, predictive analytics can forecast future trends and outcomes with remarkable accuracy. For medical writers, understanding and utilizing predictive analytics can significantly enhance their work’s quality and impact, especially in medical communication.
The Evolution of Predictive Analytics in Medical Research
Predictive analytics in medical research is not a novel concept. Traditionally, researchers have used statistical methods to analyze data and make predictions. However, the advent of AI has revolutionized this process by enabling the analysis of vast datasets with greater speed and precision. AI-powered predictive analytics can identify patterns and correlations that might be missed by human analysis, thus offering deeper insights and more accurate predictions.
Key Components of Predictive Analytics
- Data Collection and Preparation: The foundation of predictive analytics lies in collecting high-quality data. This data can come from various sources, including electronic health records (EHRs), clinical trials, and real-world evidence (RWE). Preparing this data involves cleaning, normalizing, and structuring it to ensure it is suitable for analysis.
- Model Development: AI algorithms are trained on historical data to develop predictive models. These models can range from simple linear regression to complex neural networks, depending on the nature of the data and the prediction goals.
- Validation and Testing: Before deploying predictive models, they must be validated and tested to ensure their accuracy and reliability. This involves comparing the model’s predictions with actual outcomes to assess its performance.
- Deployment and Monitoring: Once validated, predictive models are deployed in real-world settings. Continuous monitoring is essential to ensure the model remains accurate over time, and adjustments may be needed based on new data.
- Integration with Clinical Workflows:
- API development for model deployment
- User interfaces for healthcare professionals
- Integration with existing health information systems
- Continuous Monitoring and Updating:
- Performance tracking over time
- Model retraining with new data
- Drift detection and handling
- Ethical and Regulatory Compliance:
- Ensuring patient privacy and data security
- Addressing bias and fairness in AI models
- Compliance with healthcare regulations (e.g., HIPAA, GDPR)
- Domain Expertise:
- Collaboration with medical professionals
- Incorporation of clinical guidelines and medical knowledge
- Validation of AI findings against established medical practices
The Role of Predictive Analytics in Medical Communication
Enhancing Clinical Trial Reporting
One of the most significant applications of predictive analytics in medical communication is in the reporting of clinical trials. Medical writers are often tasked with summarizing complex trial data and presenting it clearly and concisely. Predictive analytics can streamline this process by:
- Disease prediction and risk assessment: AI algorithms can analyze patient data to predict the likelihood of developing certain diseases or health conditions. This helps in early intervention and preventive care.
- Drug discovery and development: AI can accelerate the drug discovery process by analyzing vast amounts of molecular and clinical data to identify potential drug candidates and predict their efficacy and safety.
- Personalized treatment plans: By analyzing patient data, genetic information, and treatment outcomes, AI can help develop personalized treatment plans tailored to individual patients.
- Medical imaging analysis: AI algorithms can analyze medical images like X-rays, MRIs, and CT scans to detect anomalies and assist in diagnosis, often catching details that human observers might miss.
- Clinical trial optimization: AI can help in patient selection for clinical trials, predict trial outcomes, and optimize trial designs, potentially reducing costs and time to market for new treatments.
- Electronic Health Record (EHR) analysis: AI can extract insights from large EHR datasets to identify patterns, predict patient outcomes, and suggest interventions.
- Epidemic prediction and management: AI models can analyze various data sources to predict disease outbreaks and help in resource allocation during epidemics.
- Biomarker discovery: AI can analyze complex biological data to identify new biomarkers for diseases, aiding in early detection and treatment monitoring.
- Healthcare resource allocation: Predictive models can help healthcare systems optimize resource allocation by forecasting patient admissions, length of stay, and required care.
- Precision medicine: AI supports the advancement of precision medicine by analyzing genetic, environmental, and lifestyle factors to predict treatment responses.
Personalizing Patient Communication
Effective patient communication is crucial in medical practice, and predictive analytics can play a pivotal role in personalizing this communication. Medical writers can leverage predictive models to:
- Tailor Information Delivery: Predictive analytics can segment patients based on their medical history, preferences, and behaviors. This allows medical writers to tailor educational materials and communication strategies to meet the specific needs of different patient groups.
- Forecasting Patient Outcomes: By analyzing patient data, predictive models can forecast individual patient outcomes, such as disease progression or response to treatment. This information can be used to create personalized care plans and educational content that addresses the unique concerns of each patient.
- Improving Engagement: Predictive analytics can identify the most effective communication channels and formats for different patient demographics. This ensures that information is delivered in a manner that maximizes patient engagement and comprehension.
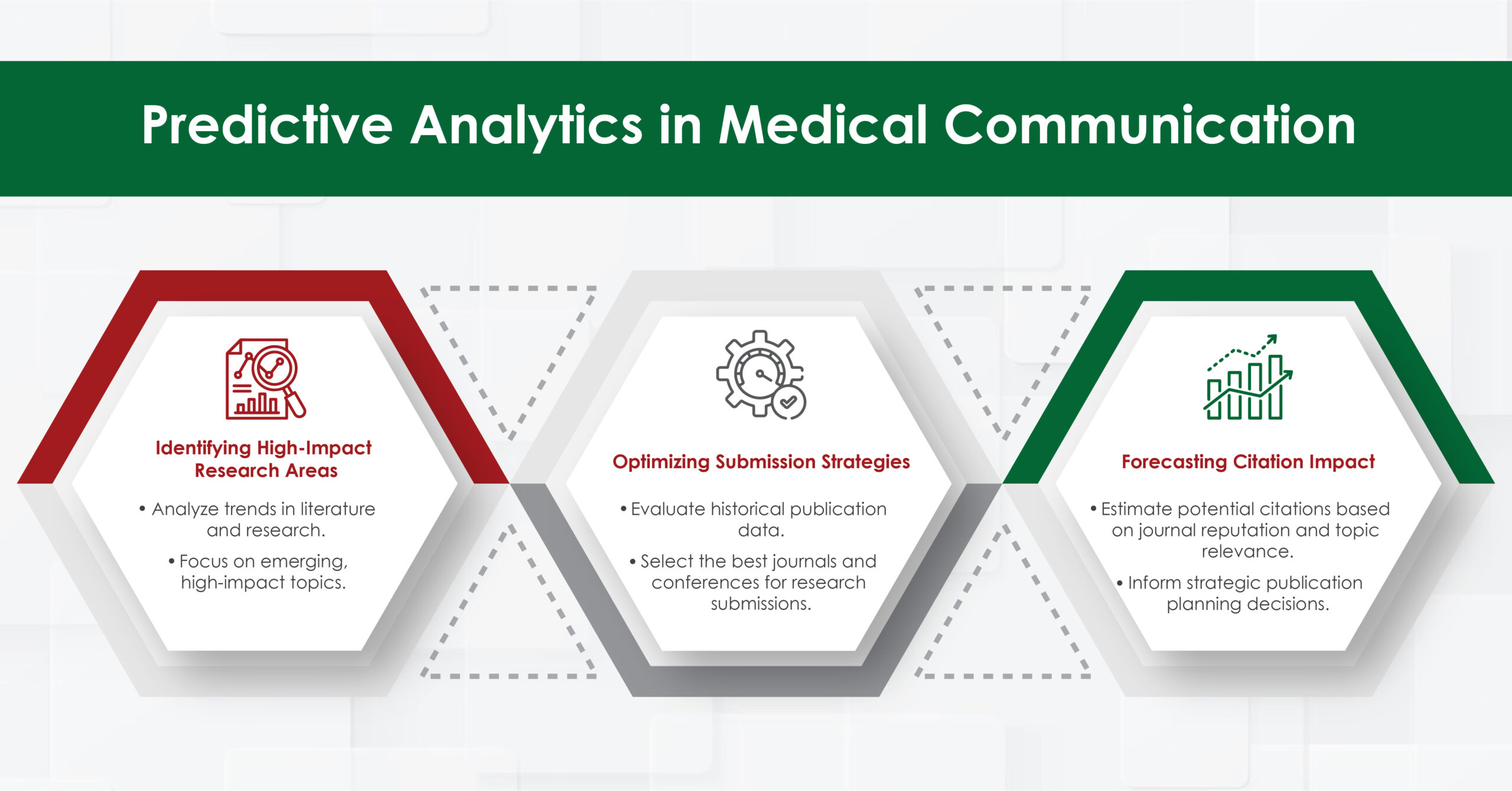
Advancing Publication Planning
In the realm of medical publication, predictive analytics can enhance the planning and execution of publication strategies. Medical writers can benefit from:
- Identifying High-Impact Research Areas: Predictive models can analyze trends in scientific literature and clinical research to identify emerging areas of interest. This helps medical writers focus on topics that are likely to have a significant impact on the field.
- Optimizing Submission Strategies: By analyzing historical publication data, predictive analytics can suggest the most appropriate journals and conferences for submitting research. This increases the likelihood of acceptance and maximizes the visibility of the work.
- Forecasting Citation Impact: Predictive models can estimate the potential citation impact of a publication based on various factors, such as the journal’s reputation and the relevance of the research topic. This information can guide strategic decisions in publication planning.
Challenges and Considerations
While the benefits of predictive analytics in medical research and communication are substantial, several challenges needs to be addressed:
- Data Quality and Integrity: The accuracy of predictive models depends heavily on the quality of the input data. Ensuring data integrity and addressing issues such as missing or biased data are critical.
- Ethical and Privacy Concerns: The use of patient data in predictive analytics raises ethical and privacy considerations. It is essential to implement robust data protection measures and adhere to ethical guidelines to maintain patient trust.
- Model Transparency and Interpretability: Complex AI models, such as deep learning algorithms, can sometimes function as “black boxes,” making it difficult to understand how they arrive at their predictions. Developing transparent and interpretable models is crucial for gaining the trust of stakeholders.
- Continuous Learning and Adaptation: Predictive models must be continuously updated with new data to maintain their accuracy. This requires ongoing investment in data collection, model development, and validation.
The Future of Predictive Analytics in Medical Communication
The integration of predictive analytics into medical research and communication is poised to grow as AI technologies advance. Staying abreast of these developments and acquiring the necessary skills to leverage predictive analytics will be essential for medical writers.
In the future, we can expect predictive analytics to become more sophisticated, offering even greater insights and more precise forecasts. This will enhance the ability of medical writers to communicate complex medical information effectively, ultimately improving patient care and advancing medical knowledge.
Predictive analytics, powered by AI, is transforming the landscape of medical research and communication. For medical writers, harnessing these technologies offers a unique opportunity to enhance the quality and impact of their work. By understanding and applying predictive analytics, medical writers can forecast trends, personalize patient communication, and optimize publication strategies, contributing to the advancement of medical science and patient care. As the field continues to evolve, embracing these tools will be crucial for staying at the forefront of medical communication.