Ovarian cancer remains one of the most challenging gynaecologic malignancies, often referred to as a “silent killer” due to its subtle onset and late-stage diagnosis.1 In 2022, 324,603 women worldwide were diagnosed with ovarian cancer. By 2050, the annual incidence is projected to rise to nearly half a million, representing a 55% increase compared to current levels.² With over 200,000 lives lost globally in 2020, this disease continues to be a significant public health concern.3 The prognosis of ovarian cancer remains disappointing, with a mere 46% of women surviving beyond 5 years after diagnosis.3 Its ability to progress without clear early symptoms makes it particularly dangerous, but early detection can dramatically improve outcomes. A powerful ally in this effort—often underrecognized—is effective medical communication.
Why Early Detection Makes All the Difference
The statistics speak for themselves. Ovarian cancer that remains localized to the ovaries (stage I) has the potential to be effectively treated in as many as 90% of patients, whereas malignancy limited to the pelvic region (Stage II) correlates with a 5-year survival rate of 70%. Conversely, neoplastic disease that has disseminated beyond the confines of the pelvis (stage III-IV) exhibits a long-term survival probability of 20% or lower.4 This stark difference underscores the importance of timely diagnosis.
From a patient’s perspective, early detection can mean a less aggressive treatment course. In some cases, surgery alone may suffice, avoiding the physical and emotional toll of chemotherapy.5 For younger women, early intervention also opens the door to fertility-preserving options.6
From a provider’s standpoint, diagnosing the disease at an early stage facilitates more personalized care, reduces treatment complications, shortens hospital stays, and ultimately improves the quality of life for patients. Additionally, early-stage management is often more cost-effective and resource-efficient, which benefits the healthcare system as a whole.
The Challenge: Recognizing the Subtle Symptoms
One of the biggest hurdles in ovarian cancer care is identifying it early. The symptoms are often vague and easily mistaken for common digestive or urinary issues. But when these signs occur frequently—12 or more times a month—they shouldn’t be ignored.7
Watch out for:
- Bloating or a swollen tummy
- Pelvic or abdominal pain
- Feeling full quickly or loss of appetite
- Needing to urinate more often or urgently
Additional symptoms may include indigestion, unintentional weight loss, changes in bowel habits (constipation or diarrhoea), back pain, fatigue, and postmenopausal vaginal bleeding.7 Although these symptoms may be dismissed as minor or attributed to other causes, persistent occurrence warrants medical evaluation.
Who’s at Greater Risk?
Several risk factors further increase susceptibility, such as:8
- Being over 50, particularly post-menopausal
- Having BRCA1 or BRCA2 gene mutations
- A family history of ovarian, breast, or colorectal cancer
- A history of endometriosis
- Never having been pregnant
- Long-term use of hormone replacement therapy
- Obesity
In individuals with these risk factors, proactive monitoring and timely screening become even more critical.
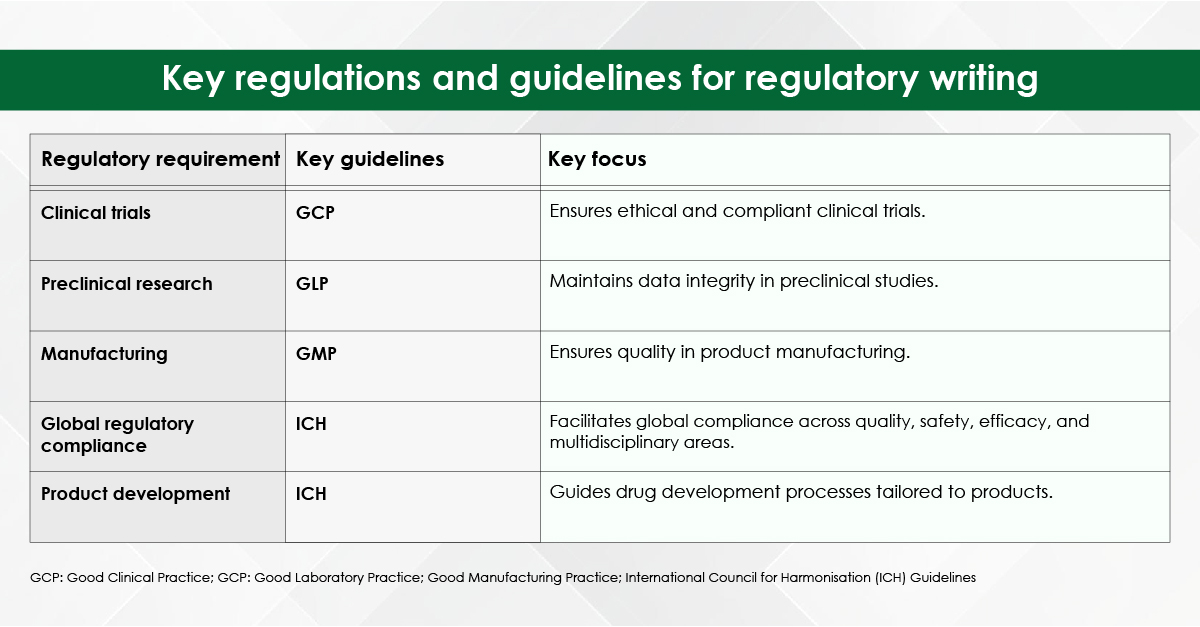
How is Ovarian Cancer Detected Early?
Unfortunately, there is no universally recommended screening test for ovarian cancer in asymptomatic women at average risk. However, several biomarkers and imaging tools are used—especially in high-risk individuals or when symptoms are present.
Key biomarkers encompass elevated levels of CA-125 (Cancer Antigen 125) and HE4 (Human Epididymis Protein). Diagnostic modalities like Risk of Ovarian Malignancy Algorithm (ROMA) integrates CA-125, HE4, and menopausal status to generate a risk score for malignancy, thereby facilitating the early detection of ovarian cancer. Furthermore, OVA1 test, a multivariate index assay, evaluates multiple protein levels in the blood to determine the risk of ovarian cancer in women presenting with an ovarian mass.8
There exist specific imaging tools that contribute to the early detection of ovarian cancer. Transvaginal ultrasound (TVUS) is instrumental in visualizing the ovaries and identifying abnormalities, such as masses or cysts, while pelvic MRI or CT scan may further assist in evaluation when ultrasound findings lack clarity.8
In women with familial predisposition to breast or ovarian cancer, genetic testing is crucial for the identification of BRCA1/2 mutations and other hereditary cancer syndromes linked to ovarian cancer. Understanding one’s genetic risk facilitates the implementation of preventive measures, including enhanced surveillance or prophylactic surgical interventions.8
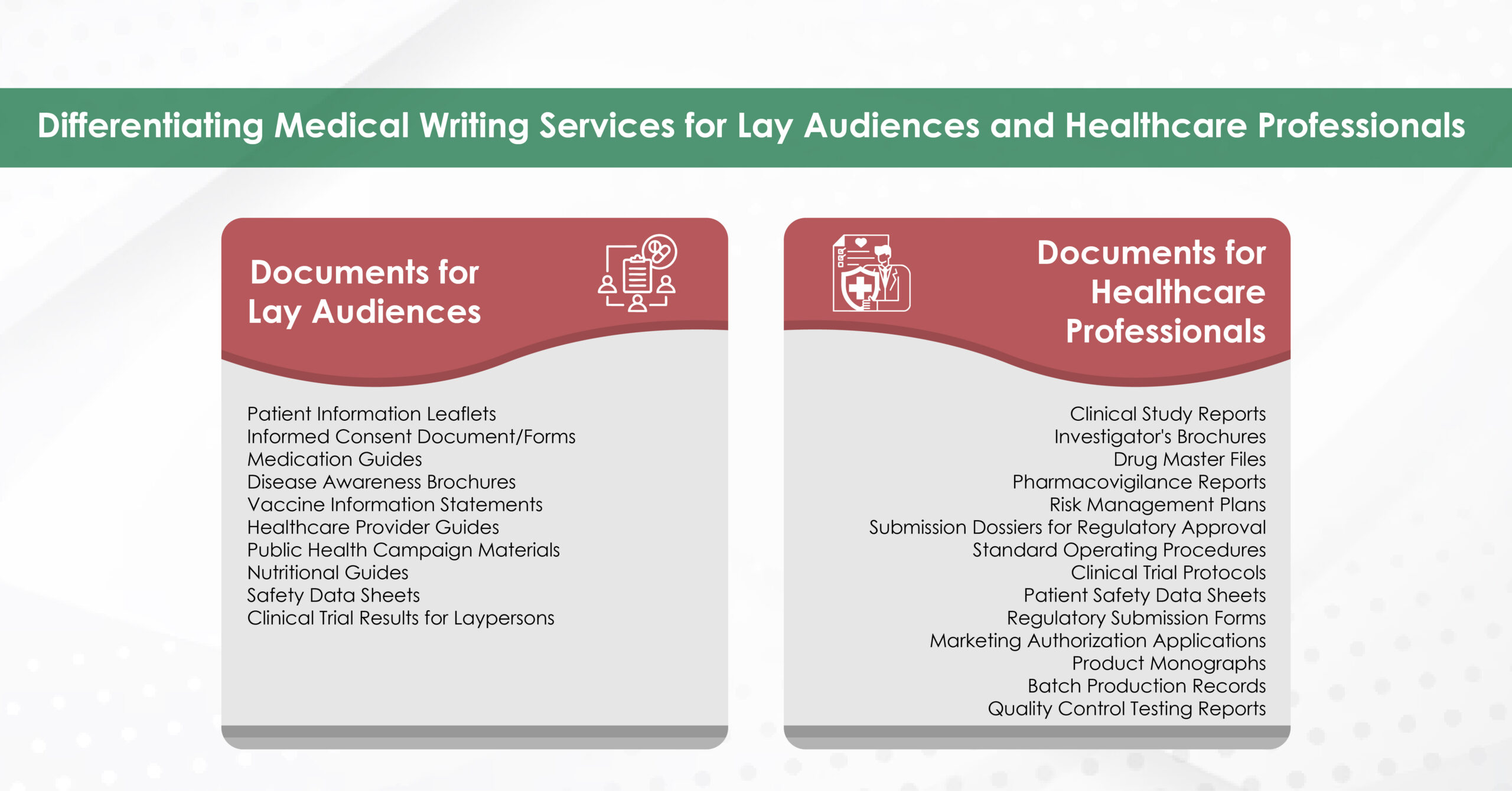
Where Medical Communication Comes In?
While awareness of symptoms and risk factors is essential, medical communication serves as the cornerstone in bridging knowledge and action. Its role is multifaceted, benefitting both patients and healthcare providers.
Empowering Patients Through Education: When patients understand early signs of ovarian cancer, they’re more likely to seek care promptly. Campaigns like the CDC’s Inside Knowledge show that clear, accessible information leads to earlier recognition and action.9
Facilitating Early Diagnosis: Empathetic, attentive dialogue from healthcare providers encourages patients to report unusual symptoms, building trust and enabling earlier detection.
Enabling Shared Decision-Making: Clear, compassionate communication helps patients understand their diagnosis, explore treatment options, and actively participate in their care—improving both outcomes and satisfaction.
Overcoming Communication Barriers: Poor communication can delay diagnosis. Culturally sensitive, respectful interactions make patients feel safe, heard, and more likely to engage in their care.
Enhancing Screening and Prevention: Regular discussions about risk, symptoms, and family history motivate patients to adopt preventive behaviours and pursue early screening—critical for early-stage detection.
The Role of Medical Communication Agencies
At every stage—from raising awareness to supporting diagnosis, treatment decisions, and prevention—medical communication agencies act as strategic partners in improving outcomes. By transforming complex medical information into patient-friendly content, developing provider training tools, and designing culturally sensitive campaigns, these agencies ensure that critical health messages resonate. Their expertise in crafting clear, compassionate, and actionable communication bridges the gap between medical knowledge and patient engagement, ultimately driving earlier detection and better care experiences across the board.
Conclusion
Ovarian cancer remains challenging due to its silent onset and rapid progression, but early detection can shift outcomes dramatically. Medical communication is not just supportive—it’s a strategic enabler, driving awareness, encouraging timely symptom reporting, and building trust between patients and providers. As diagnostics advance, the impact of clear, compassionate communication remains vital in bridging the gap between symptom onset and timely intervention.
Turacoz specializes in in transforming complex medical information into clear, actionable content that supports both patients and healthcare providers. From developing symptom awareness tools to creating dialogue aids and educational campaigns, we enable early recognition, informed decision-making, and timely action. With expertise in oncology communication, we craft materials that foster trust, encourage preventive behaviour, and enhance patient-provider conversations—ultimately contributing to earlier detection and improved outcomes in ovarian cancer care.
References
- Feeney L, et al. Liquid biopsy in ovarian cancer: Catching the silent killer before it strikes. World J Clin Oncol. 2020;11(11):868-889.
- World Ovarian Cancer Coalition. Available at: https://worldovariancancercoalition.org/wp-content/uploads/2024/04/2024-Global-Priority.pdf. Last accessed: May 2025.
- Wang M, Bi Y, Jin Y, and Zheng ZJ. Global Incidence of Ovarian Cancer According to Histologic Subtype: A Population-Based Cancer Registry Study. JCO Glob Oncol. 2024;10:e2300393.
- Elias KM, Guo J, and Bast RC Jr. Early Detection of Ovarian Cancer. Hematol Oncol Clin North Am. 2018;32(6):903-914.
- Fishman DA and Bozorgi K. The scientific basis of early detection of epithelial ovarian cancer: the National Ovarian Cancer Early Detection Program (NOCEDP). Cancer Treat Res. 2002;107:3-28.
- Kim SY and Lee JR. Fertility preservation option in young women with ovarian cancer. Future Oncol. 2016 Jul;12(14):1695-8.
- Ovarian cancer. Available at: https://www.nhs.uk/conditions/ovarian-cancer/symptoms/. Last accessed: May 2025.
- Fernandes B. A Review on Emerging Biomarkers for Early Detection of Ovarian Cancer. Ind J Pharm Pract. 2024;17(1):17–20.
- Cooper CP, Polonec L, and Gelb CA. Women’s knowledge and awareness of gynecologic cancer: a multisite qualitative study in the United States. J Womens Health (Larchmt). 2011;20(4):517-524.