
The healthcare industry is experiencing a paradigm shift as patient voices take center stage in drug development and approval processes. While clinical trial data continues to be the cornerstone of drug development and approval, patient-reported outcomes (PROs) are emerging as the gold standard for demonstrating real-world value, particularly when it comes to market access approvals offering valuable insights into the patient experience and treatment impact. As healthcare systems worldwide shift toward value-based care, PROs are getting more and more widely used in clinical trials and approval processes. For example, the proportion of industry-sponsored oncology trials including PROs assessments rose from 26% (2007–2013) to 75% (2014–2018).1 This increased integration of PROs into clinical trials and regulatory submissions reflects a growing acknowledgment of their value in evaluating therapies from the patient’s perspective.
The Evolution of Healthcare Metrics
Historically, drug approvals focused primarily on “hard” clinical endpoints, while these metrics remain important, they tell only part of the story, and do not completely capture the picture of quality of life (QoL) or daily functioning. PROs that reflect the patient’s direct perspective on their symptoms, functional status, and overall well-being capture this crucial dimension that clinical data alone cannot measure complementing the traditional clinical outcomes. This holistic view of treatment effects is particularly important for chronic and debilitating conditions, where symptom burden and QoL are critical determinants of treatment success.2,3
Regulatory Recognition
Regulatory bodies worldwide have recognized this gap and are increasingly demanding PRO data as part of approval submissions:
The increasing integration of PROs into regulatory submissions underlines their significance in demonstrating treatment benefits from a patient perspective.
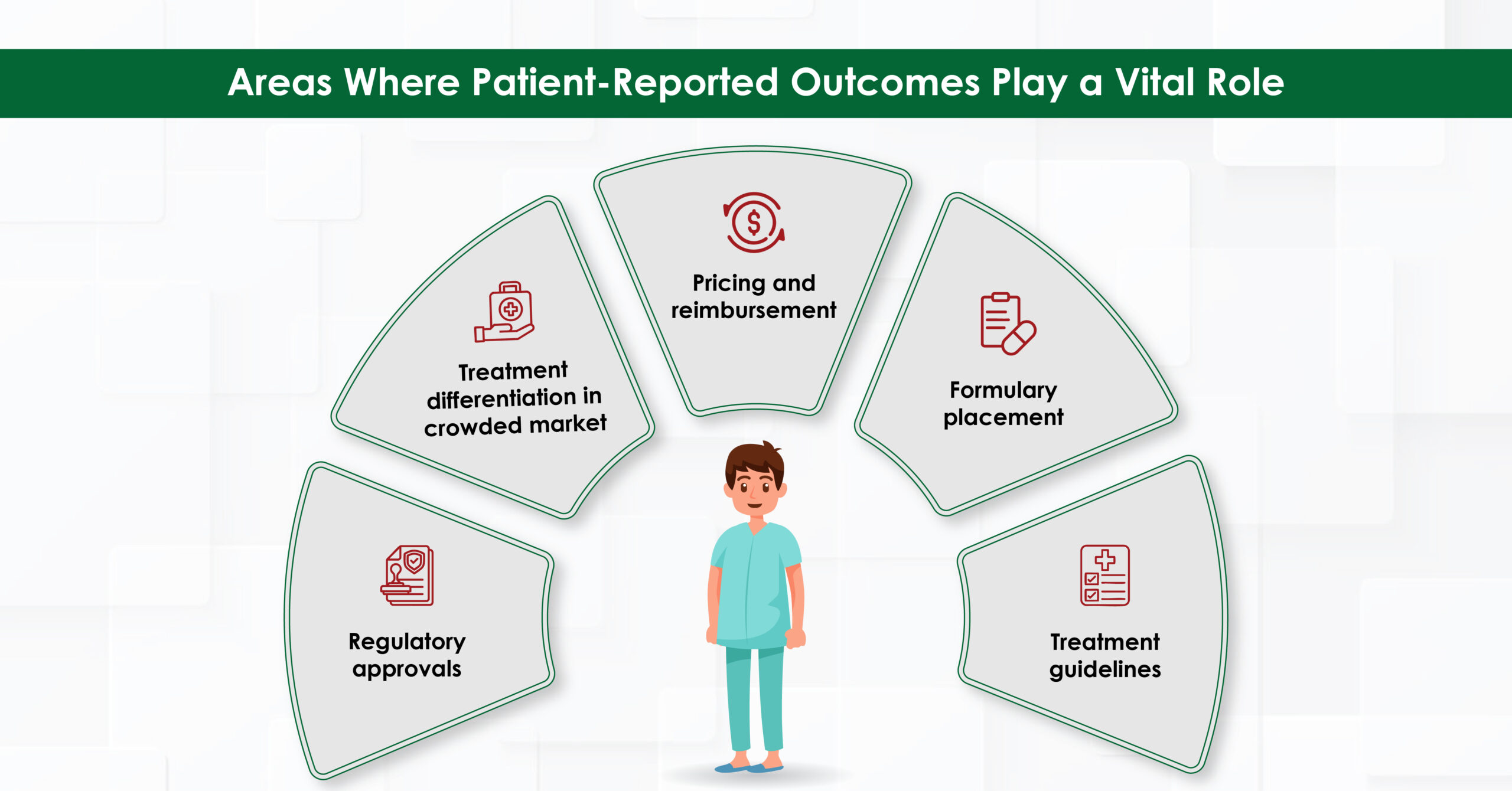
The Market Access Imperative
For pharmaceutical companies PRO data is becoming essential for market access success. Here’s why:
Differentiation in Crowded Markets
PRO data can help to distinguish therapies, especially in oncology post-progression scenarios. A study has indicated that positive PRO data such as superior symptom relief, improved physical functioning support continued therapy at the physician’s discretion upon regulatory approval, even in progressive disease.7
Pricing and Reimbursement Leverage
The U.S. healthcare system is shifting from fee-for-service to value-based payment models to enhance patient care quality and control costs. Under the 2015 Medicare Access and Children’s Health Insurance Program Reauthorization Act, providers will be assessed on quality and cost efficiency, affecting their reimbursement rates. PROs play a key role in this transition by offering insights into patient preferences, experiences, and perceptions of benefits and risks. These insights inform pricing, reimbursement, and benefit-risk assessments, ensuring treatments align with patient values. PROs also influence health technology assessments by evaluating the impact of medical technologies on quality of life, guiding more equitable pricing decisions based on what patients value.8,9
Formulary Placement and Treatment Guidelines
Clinical practice guidelines are giving greater weight to PROs evidence when making recommendations. For example, European Society for Medical Oncology (ESMO) recommends symptom monitoring using patient-reported outcome measures (PROMs) for patients with stage IIIB/IV lung cancer who have completed initial or maintenance treatment. Additionally, it also recommends PROMs in survivorship care of patients after treatment of cancer, to improve communication and identify late toxicities, symptoms or functional impairment warranting supportive care.10
Beyond regulatory initiatives, incorporating PROs can increase the “value” of your therapeutic from a payor perspective, ultimately helping formulary placement.11
Advances in Digital Data Collection Have Made Collecting PROs Easy
The advent of digital health technologies has facilitated the collection and analysis of PROs, making them more accessible and actionable. Electronic patient-reported outcome measures (ePROMs) enable real-time data capture, reducing barriers to implementation and improving the quality of PRO data.6,12 For example, digital platforms are being used to collect PROs in large-scale studies, such as the PROMchronic study in Germany, which aims to evaluate the effectiveness of ePROMs in improving care for patients with chronic diseases like diabetes and asthma.6 Additionally, AI and machine learning help analyze PRO data to identify patterns and insights.
Challenges and Opportunities
Despite their growing importance, the use of PROs in market access approvals is not without challenges. Issues such as the lack of standardization, variability in data quality, and the need for robust methodologies remain. However, ongoing research and policy initiatives are addressing these challenges, with a focus on developing validated instruments, improving data collection practices, and integrating PROs into regulatory frameworks.13,14
For example, the European Medicines Agency (EMA) has emphasized the need for harmonization of PRO measures to facilitate their use in drug development and regulatory decision-making. Similarly, initiatives like the Innovative Medicines Initiative (IMI) PREFER project are working to establish best practices for incorporating patient preferences into regulatory evaluations.14,15
The Future of PROs in Market Access
The future of PROs in market access approvals is promising, with ongoing advancements in technology, policy, and methodology. As regulators and payers increasingly recognize the value of patient-centered data, PROs are likely to become even more integral to healthcare decision-making. Their ability to capture the patient’s perspective, complement traditional outcomes, and support real-world evidence makes them indispensable in the era of value-based healthcare.16
Conclusion
In conclusion, PROs are becoming the gold standard for market access approvals because they provide a patient-centered perspective, complement traditional clinical outcomes, and support regulatory and reimbursement decisions with real-world evidence. As healthcare systems continue to evolve, the integration of PROs into decision-making processes will remain a cornerstone of value-based, patient-centered care.
References