Academic publishing is undergoing a significant transformation, driven by technological advancements, changing attitudes toward knowledge dissemination, and the need for greater transparency in research. As medical writers, it’s crucial to stay informed of these developments to better serve our clients and contribute to the evolving scholarly communication ecosystem. This blog explores emerging trends and predicts future developments in academic publishing, with a focus on their implications for medical writing.
Open Peer Review: Transparency in the Evaluation Process
One of the most notable trends in academic publishing is the move toward open peer review. Traditionally, peer review was typically a closed process, with reviewers remaining anonymous and their comments hidden from public view. However, there’s a growing push for transparency in this crucial step of scholarly publishing.
Open peer review can take various forms, ranging from simply publishing reviewer reports alongside the final article to revealing reviewer identities and allowing public comment on preprints. This shift towards openness aims to address several issues in the current system, including:
- Accountability: By making reviewer comments public, there is increased accountability for both reviewers and authors.
- Credit for Reviewers: Open peer review allows reviewers to receive recognition for their contributions to the scientific process.
- Educational Value: Early career researchers can learn from seeing high-quality peer reviews.
- Reducing Bias: Open peer review may help diminish some forms of bias in the review process.
For medical writers, this trend necessitates a deeper understanding of the peer review process and the ability to guide clients through more transparent scholarly communication. We may need to assist authors in preparing responses to reviewer comments that will be publicly visible, requiring a more tactful and constructive approach.
Data Sharing Policies: Enhancing Reproducibility and Transparency
Another significant trend is the implementation of stricter data-sharing policies by journals and funding bodies to promote open data. This push aims to enhance research reproducibility, allow for secondary analyses, and increase overall transparency in scientific research.
Key aspects of this trend include:
- Mandatory Data Availability Statements: Many journals now require authors to include a statement on how and where their data can be accessed, fostering data transparency.
- Data Repositories: The use of specialized repositories for different types of data (e.g., genomic, imaging, clinical trial data) is becoming more common.
- FAIR Principles: There is an increasing emphasis on making data Findable, Accessible, Interoperable, and Reusable, promoting data usability and accessibility.
For medical writers, this trend requires a thorough understanding of data management practices and the ability to guide clients in preparing their data for sharing. We may need to assist in writing clear data availability statements, ensuring proper data anonymization, and navigating the complexities of various data-sharing platforms.
Artificial Intelligence in Publishing: Shaping the Future
Artificial Intelligence (AI) is poised to revolutionize various aspects of academic publishing. While it will not replace human expertise, AI will likely augment and streamline many processes. Some key areas where AI is making inroads include:
- Manuscript Screening: AI tools can help editors quickly assess whether submissions meet basic criteria and are within the journal’s scope.
- Plagiarism Detection: Advanced AI algorithms can detect not just verbatim copying but also paraphrased content and idea plagiarism.
- Reference Checking: AI can verify the accuracy and completeness of citations more efficiently than manual checking.
- Language Polishing: AI-powered tools can assist in improving the clarity and grammar of manuscripts, particularly beneficial for non-native English speakers.
- Peer Reviewer Matching: AI algorithms can suggest appropriate reviewers based on the manuscript’s content and reviewers’ expertise.
As medical writers, it is essential to stay informed about these AI tools and potentially incorporate them into our workflow. However, it is crucial to maintain a critical eye and not over-rely on AI-generated content or suggestions.
Preprint Servers: Accelerating Scientific Communication
The rise of preprint servers, such as medRxiv for health sciences, is another trend reshaping academic publishing. These servers allow researchers to share their findings rapidly, before the often lengthy peer review process. This trend has several implications:
- Faster Dissemination of Research: Crucial in fast-moving fields or during health crises like the COVID-19 pandemic.
- Increased Visibility: These can attract collaborators and feedback early in the research process.
- Establishing Priority: Researchers can stake their claim to ideas and findings earlier.
However, the proliferation of preprints also raises concerns about the quality of non-peer-reviewed research entering the public domain. As medical writers, we may need to help clients navigate the decision of whether to post preprints and assist in preparing manuscripts that clearly state their preprint status.
Predictions for the Future of Academic Publishing
Looking ahead, several developments are likely to shape the future of academic publishing:
- Blockchain for Peer Review: Blockchain technology could be used to create a transparent, immutable record of the peer review process, potentially addressing issues of trust and accountability.
- AI-Assisted Authorship: While AI will not replace human authors, it may play a larger role in literature reviews, generating hypotheses, and even drafting sections of papers.
- Interactive Papers: Future academic papers may incorporate multimedia elements, live data visualizations, and even virtual reality components to create a more interactive reading experience.
- Micro-publications: There may be a shift towards publishing smaller units of research, such as individual experiments or observations, rather than waiting to compile a full paper.
- Continuous Publishing: Some journals may abandon the issue and volumes-based model, instead publish articles on a rolling basis as soon as they are ready.
- Alternative Metrics: Traditional impact factors may be supplemented or replaced by more diverse metrics that capture societal impact, policy influence, and public engagement.
- Open Access Dominance: The trend towards open access is likely to continue, potentially becoming the dominant model for academic publishing, with more journals making their content freely available online.
Peer Review Evolution: These may become more collaborative, with reviewers and authors working together to improve papers through multiple rounds of feedback.
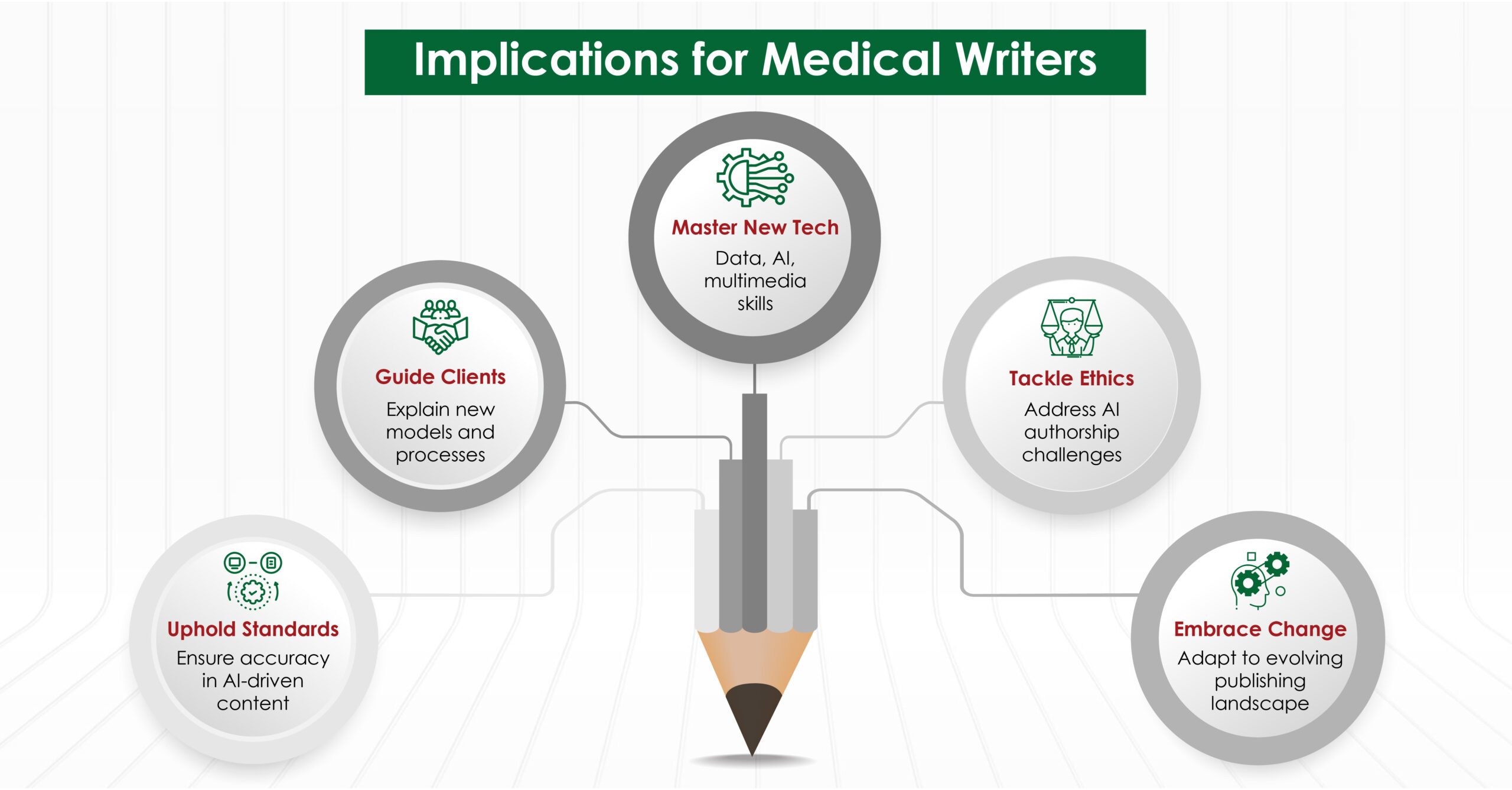
Implications for Medical Writers
These trends and predictions have significant implications for medical writers:
- Expanded Skill Set: We will need to develop new skills, such as data management, AI tool utilization, and multimedia content creation.
- Ethical Considerations: As AI becomes more prevalent in writing and publishing, we willneed to navigate complex ethical issues around authorship and originality.
- Adaptability: The publishing landscape is likely to remain in flux, requiring us to stay adaptable and continuously update our knowledge and practices.
- Client Education: We will play an important role in educating clients about new publishing models, data-sharing requirements, and the evolving peer review process.
- Quality Assurance: With the rise of preprints and AI-assisted writing, our role in ensuring the quality and accuracy of scientific communication will become even more crucial.
- Interdisciplinary Collaboration: As research becomes more complex and data-intensive, we may need to collaborate more closely with data scientists, statisticians, and other specialists.
The future of academic publishing is exciting and challenging, with trends towards greater openness, transparency, and technological integration. As medical writers, we are uniquely positioned to help navigate this changing landscape. By staying informed about these trends and developing new skills, we can continue to play a vital role in ensuring effective and accurate scientific communication.
The key to thriving in this evolving environment will be our ability to adapt, embrace new technologies while maintaining a critical perspective and continue to prioritize the clear and accurate communication of scientific ideas. As we move forward, our expertise in crafting compelling narratives and translating complex scientific concepts will remain invaluable, even as the medium and methods of publishing continue to evolve.
Turacoz Healthcare Solutions stands at the forefront of academic publishing, offering comprehensive medical writing services that cater to the needs of researchers, clinicians, and academicians. Our team is adept at navigating the complexities of modern publishing, from open peer review to AI integration and data sharing. Partnering with Turacoz, you gain access to a wealth of knowledge and experience to help you publish confidently and clearly. Visit www.turacoz.com or contact us at [email protected] to learn more about how we can support your academic publishing journey.