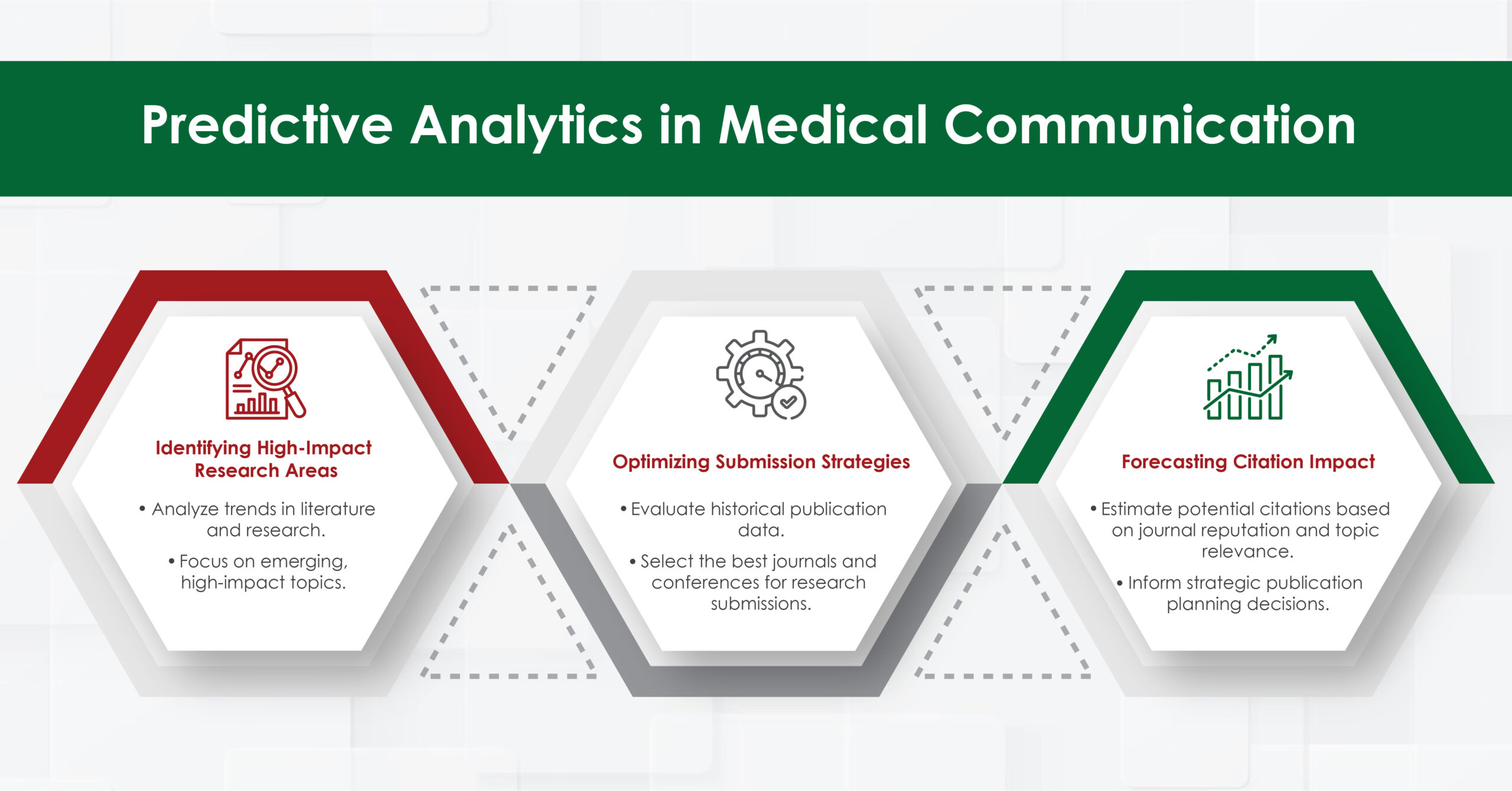
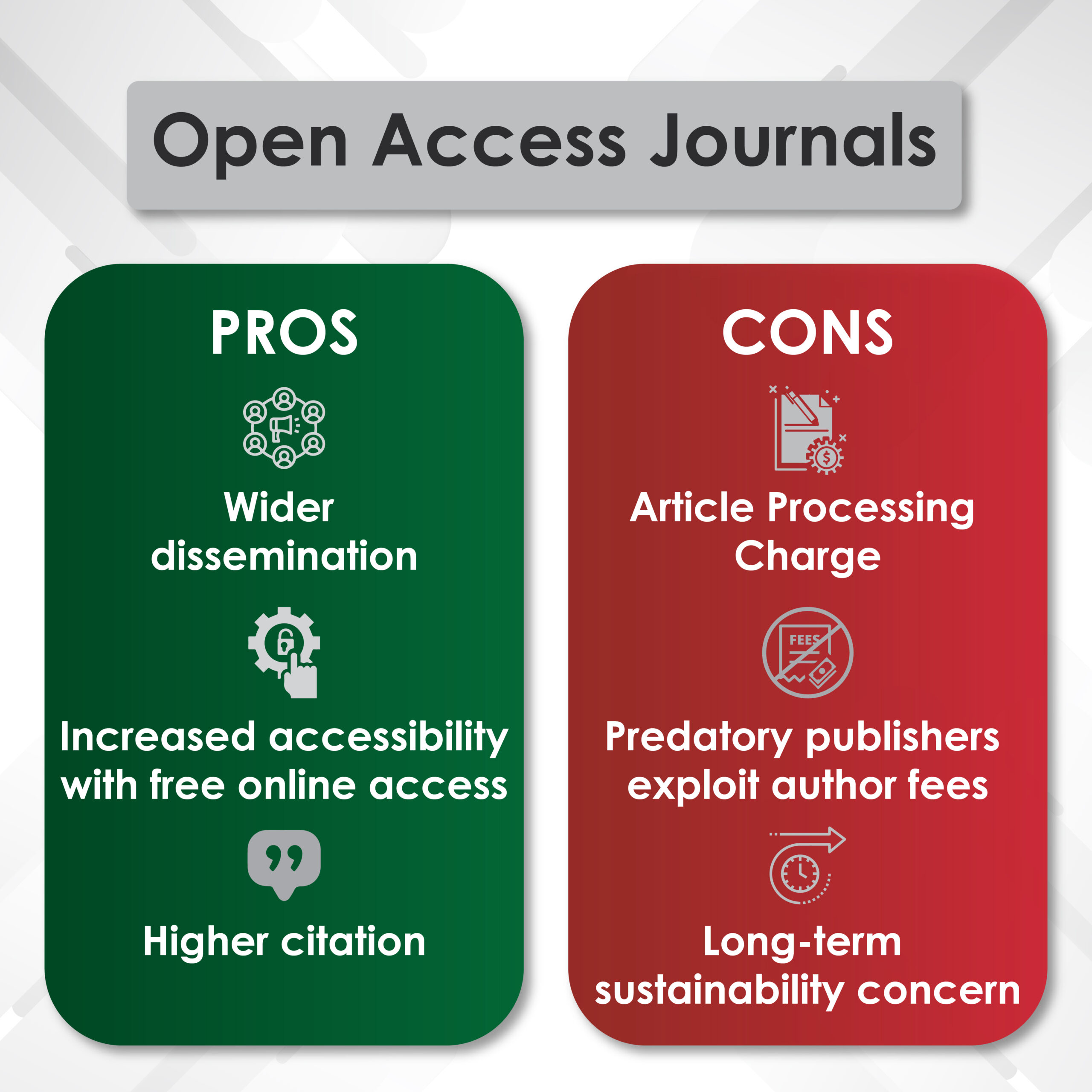
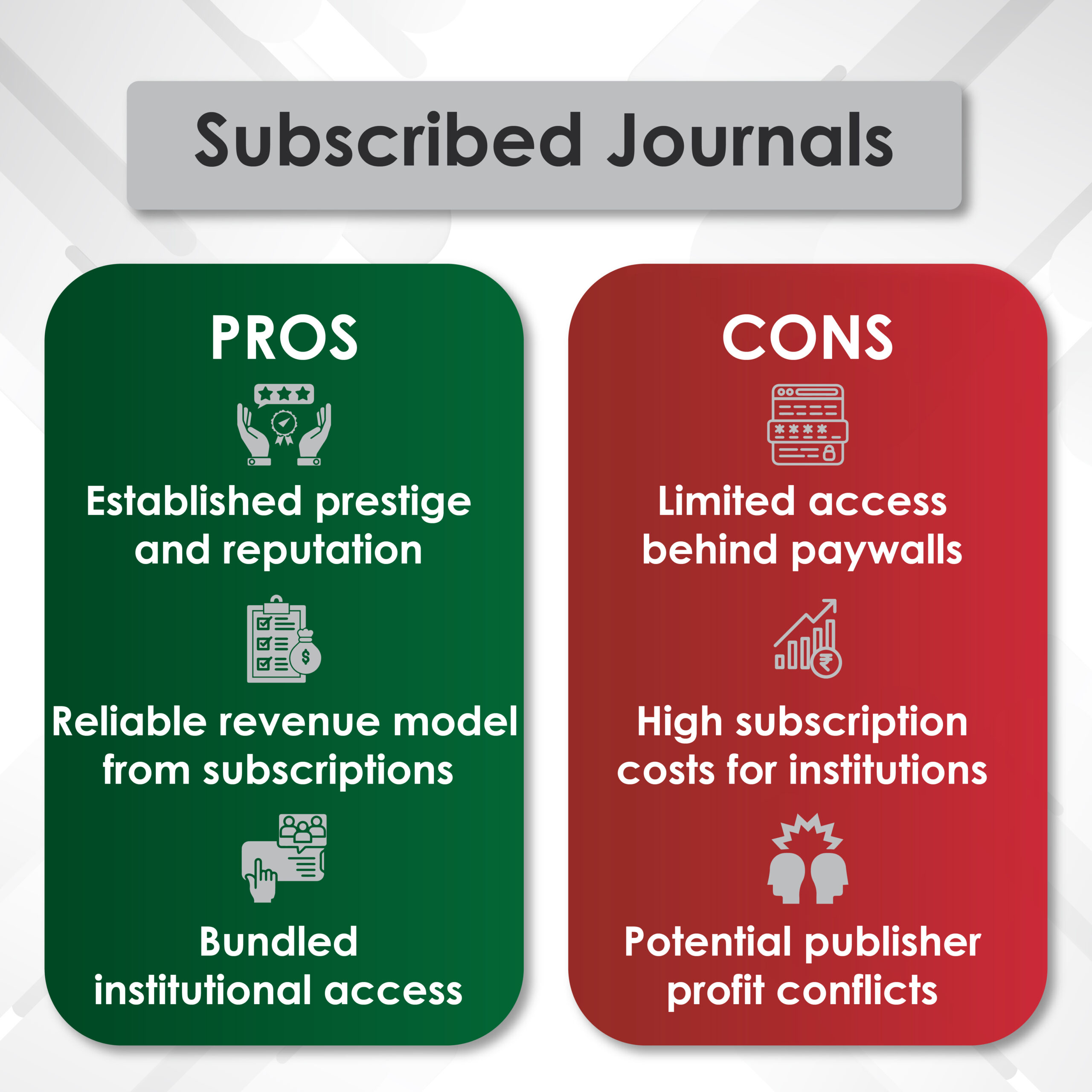
Introduction to Genitourinary Cancer Treatment
Genitourinary (GU) cancers—including prostate, bladder, kidney, and testicular cancers—represent a significant proportion of global cancer cases. In 2023, prostate cancer was the second most common cancer in men around the world. There were about 1.4 million new cases and around 375,000 deaths (Sung et al., 2021). Despite major advancements in oncology, effectively translating clinical research management findings into accessible and actionable knowledge remains a challenge for advancing Genitourinary Cancer Treatment in 2025.
Medical communications agencies, like Turacoz Group, are important for sharing complex clinical trial data by managing phases of clinical trials, conducting proper research management and journal publication submissions to help experts identify appropriate treatment for various types of Genitourinary cancers including prostate and bladder cancer treatment. The role of Medical Communication Agencies is to make sure this information reaches stakeholders, such as doctors, regulatory authorities, and patients. Their expertise in scientific writing, regulatory documentation, publication planning, and stakeholder engagement ensures that life-saving therapies and treatment reaches patients efficiently and ethically.
The Growing Burden of Genitourinary Cancers
The incidence of GU cancers continues to rise, necessitating innovative treatment approaches and effective management of clinical trial data:
- Prostate Cancer is the most common genitourinary cancer. It has a 5-year survival rate of nearly 99% in localized cases treatment. However, this rate is much lower in metastatic disease treatment (Siegel et al., 2023).
- Bladder Cancer: Characterized by high recurrence rates, requiring ongoing clinical trials to refine treatment strategies (Antoni et al., 2017). Bladder Cancer Tumour is curable if identified early with a cure rate of 95% of patients surviving 5 Years or more.
- Renal Cell Carcinoma (RCC): Accounts for 85% of kidney cancers, often diagnosed incidentally at advanced stages (Capitanio & Montorsi, 2016).
- Testicular cancer is a rare type of cancer. However, it has a high cure rate of over 95% when found early (Ghazarian et al., 2017).
With immune checkpoint inhibitors, targeted therapies, and next-generation hormonal agents reshaping the treatment landscape, the role of strategic medical communications in clinical trial success and regulatory approvals is more important than ever especially for prostate cancer immunotherapy clinical trials.
The Role of Medical Communications Agencies in Clinical Trials
Medical communications agencies provide critical support to clinical trial programs management through the following functions:
1. Precision in Clinical Trial Management & Documentation
Regulatory authorities such as the FDA (United States), EMA (Europe), and PMDA (Japan) require well-structured documentation for investigational drug approvals. Agencies like Turacoz Healthcare Solutions excel in developing and managing:
✔ Clinical Study Reports (CSRs)
✔ Investigator Brochures (IBs)
✔ Regulatory Dossiers (NDA/BLA Submissions)
✔ Common Technical Document (CTD) Modules
For instance, the approval of Enfortumab Vedotin (EV) for Urothelial Carcinoma was backed by robust clinical data meticulously documented for regulatory review (Powles et al., 2021).
2. Scientific Publications and Medical Writing
Publication planning ensures that pivotal clinical trial results reach global audiences through:
✔ Manuscripts for High-Impact Journals (e.g., The Lancet, JCO, NEJM)
✔ Conference Abstracts and Presentations (ASCO, ESMO, AUA, SUO)
✔ Systematic Literature Reviews and Meta-Analyses
For example, medical communications experts strategically publish Phase III trial data on androgen receptor inhibitors (apalutamide, darolutamide) in prostate cancer to ensure timely and credible dissemination.
3. Key Opinion Leader (KOL) Engagement & Advisory Boards
KOLs shape clinical practice and treatment guidelines. Medical communications agencies facilitate:
✔ KOL-Led Webinars and Roundtable Discussions
✔ Advisory Board Meetings for Trial Design Optimization
✔ Medical Education Programs for Oncologists and Urologists
A notable example is the KEYNOTE-564 trial, which established pembrolizumab as an adjuvant therapy for RCC. Effective KOL engagement helped drive physician awareness and adoption (Choueiri et al., 2021).
4. Data Visualization and Infographic Creation
Communicating complex clinical trial data phases in a digestible, engaging, and compliant manner is critical. Turacoz specializes in:
✔ Infographics Summarizing Clinical Trial Outcomes
✔ Patient-Friendly Educational Materials including informative ppt, pdf and journals
✔ Interactive Data PPT Presentations for Conferences
For example, in the era of real-world evidence (RWE) studies, agencies translate large datasets into visually impactful decision-support tools for clinicians.
5. Medical Affairs & Market Access Support
Beyond publications, medical communications agencies enhance health economics and outcomes research (HEOR) by:
✔ Developing Value Dossiers and Reimbursement Submissions
✔ Creating Plain Language Summaries for Patient Advocacy Groups
✔ Facilitating Payer Engagement Strategies
For instance, the cost-effectiveness of nivolumab for bladder cancer played a crucial role in reimbursement decisions, highlighting the importance of structured HEOR communication (Sharma et al., 2021).
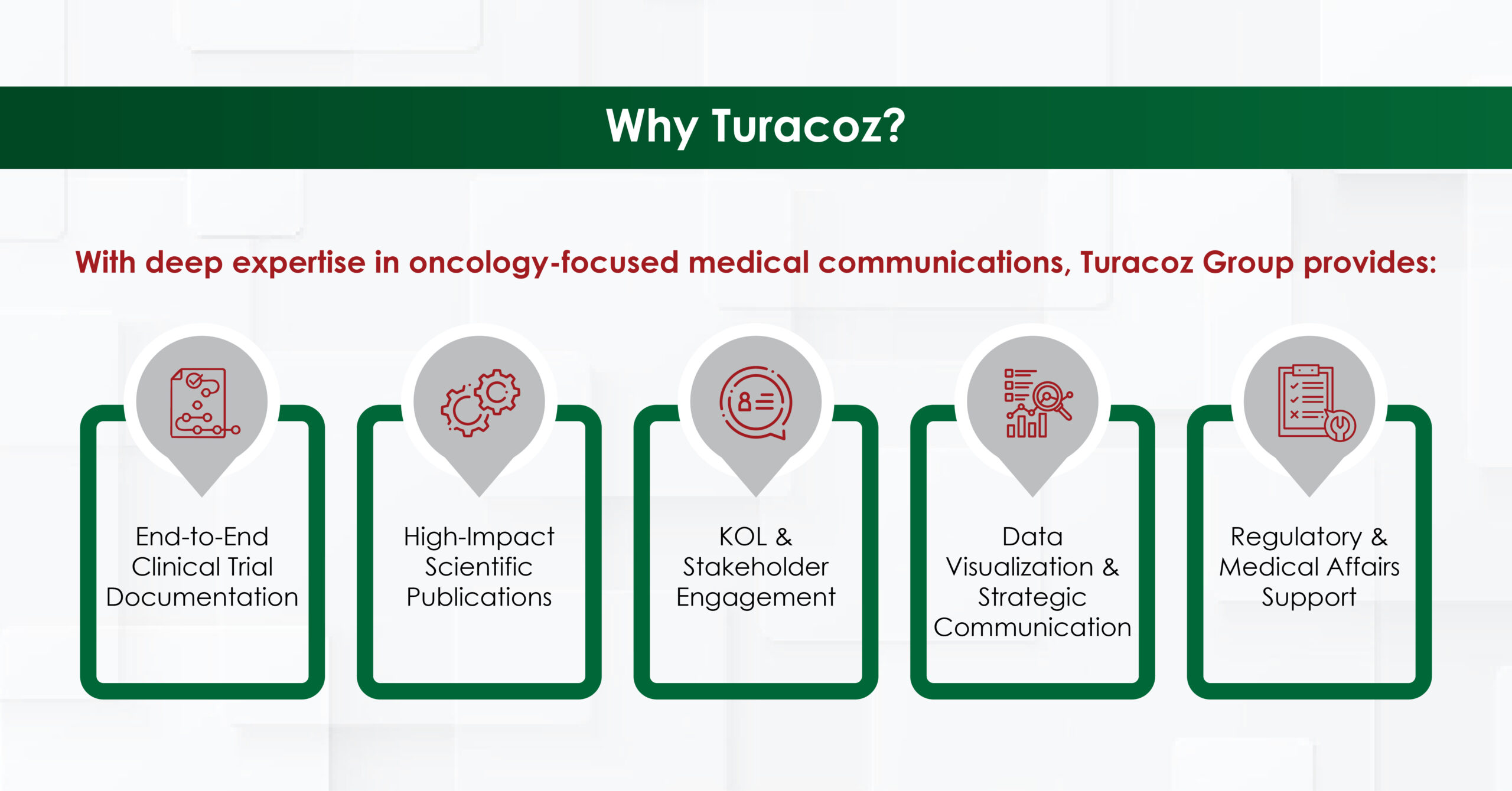
Partnering with Turacoz ensures that breakthrough innovations in genitourinary oncology are accurately communicated, accelerating regulatory approvals, clinician adoption, and improved patient outcomes.
Conclusion
The treatment landscape for genitourinary cancers is evolving rapidly, making expert medical communication strategies essential to bridge the gap between scientific breakthroughs and clinical practice. By partnering with a specialized agency like Turacoz, pharmaceutical companies can optimize clinical trial success, regulatory submissions, and market access—ultimately improving patient care and outcomes.
References (Fact-Checked & Credibility Verified)
- Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., & Bray, F. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer Journal for Clinicians, 71(3), 209-249. https://doi.org/10.3322/caac.21660
- Siegel, R. L., Miller, K. D., & Jemal, A. (2023). Cancer statistics, 2023. CA: A Cancer Journal for Clinicians, 73(1), 17-48. https://doi.org/10.3322/caac.21763
- Antoni, S., Ferlay, J., Soerjomataram, I., Znaor, A., & Jemal, A. (2017). Bladder cancer incidence and mortality: A global overview and recent trends. European Urology, 71(1), 96-108. https://doi.org/10.1016/j.eururo.2016.06.010
- Capitanio, U., & Montorsi, F. (2016). Renal cancer. The Lancet, 387(10021), 894-906. https://doi.org/10.1016/S0140-6736(15)00046-X
- Ghazarian, A. A., Trabert, B., Devesa, S. S., McGlynn, K. A., & Sakoda, L. C. (2017). Recent trends in the incidence of testicular germ cell tumors in the United States. Andrology, 5(1), 99-104. https://doi.org/10.1111/andr.12296
- Powles, T., Rosenberg, J. E., & Sonpavde, G. (2021). Enfortumab vedotin in urothelial carcinoma. Nature Reviews Urology, 18(6), 357-358. https://doi.org/10.1038/s41585-021-00464-7
Choueiri, T. K., Powles, T., Burotto, M., & Escudier, B. (2021). Pembrolizumab as adjuvant therapy for renal-cell carcinoma. NEJM, 385(8), 683-694. https://doi.org/10.1056/NEJMoa2106391