In the evolving medical research field, identifying unexplored areas and novel opportunities is crucial for advancing scientific knowledge and improving patient outcomes. Effective, traditional methods of literature review and gap analysis can often be time-consuming and prone to human error. This is where artificial intelligence (AI) – a transformative technology- plays a key role in revolutionizing how researchers identify gaps in the literature and uncover new avenues for investigation. This blog explores the role of AI in medical research, specifically how it can analyze existing literature to identify research gaps and suggest new opportunities.
Role of AI in Medical Research
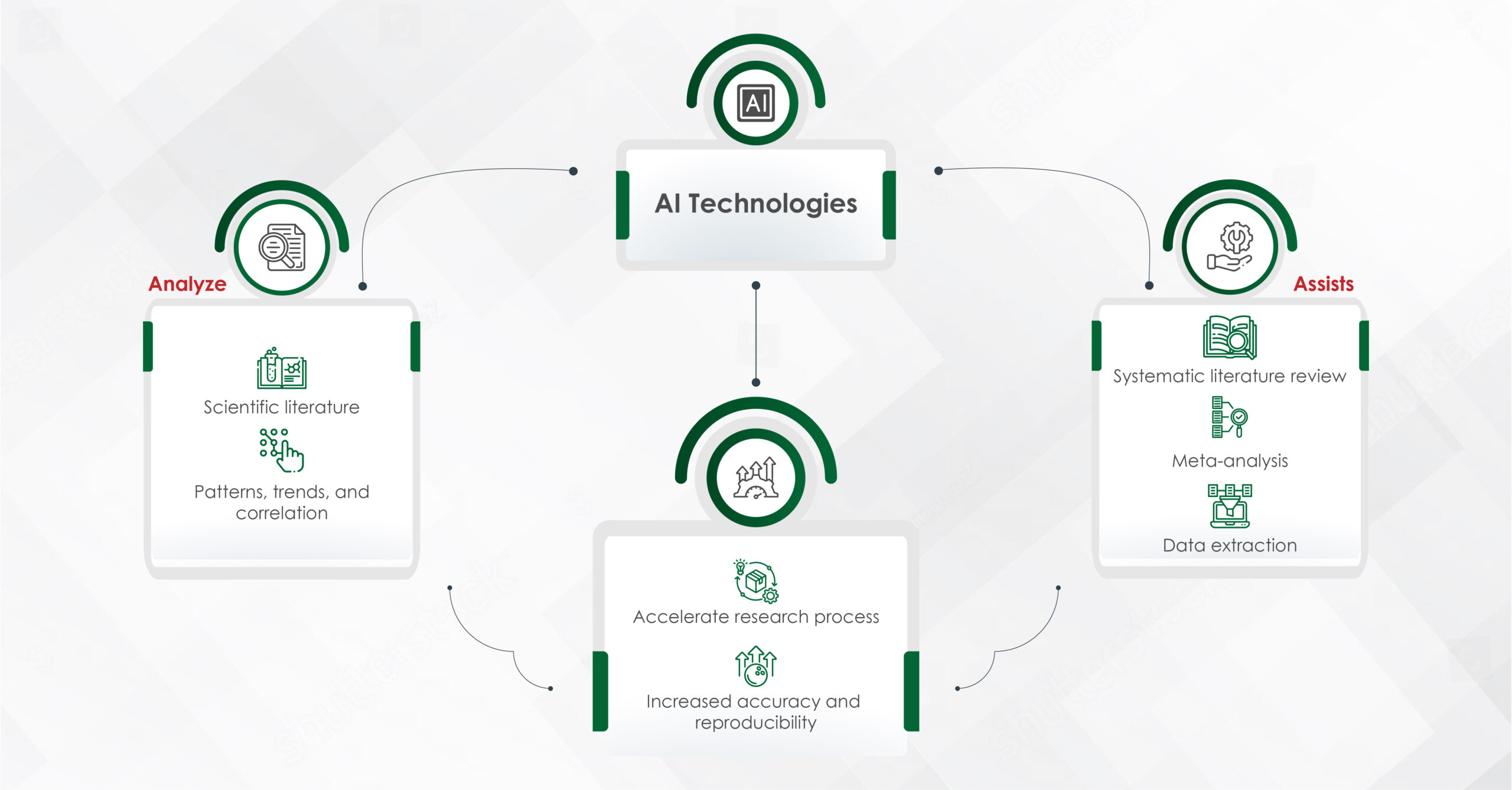
Artificial intelligence, with its capacity to process vast amounts of data quickly and accurately, offers a powerful tool for researchers. AI technologies, such as machine learning (ML) and natural language processing (NLP), can scan and analyze thousands of research papers, clinical trials, and medical records, providing insights that would be impossible to achieve manually.
One of the primary applications of AI in medical research is in literature mining. Systematic literature reviews (SLRs) and meta-analyses, in particular, are critical for synthesizing existing knowledge. However, conducting an SLR manually can take several months to over a year, requiring researchers to sift through thousands of articles to identify relevant studies. This laborious process often involves multiple rounds of selection and data extraction. With AI tools like Covidence, Rayyan, Easy SLR, and Robot Reviewer, this timeline can be drastically reduced, as AI automates the initial stages of searching, screening, and extracting data from large datasets, making the process more efficient.
Moreover, AI can assist in meta-analyses by automating the extraction of relevant data from studies, calculating effect sizes, and synthesizing findings. This automation not only accelerates the research process but also enhances the accuracy and reproducibility of the results.
AI in Identifying Research Gaps
The identification of research gaps is a critical step in the scientific process. A research gap represents an area within a field where little or no information is available, indicating a need for further study. Traditionally, identifying these gaps required extensive literature review, expert consultation, and a deep understanding of the field. However, AI offers a more efficient and systematic approach.
- Automated Literature Review
AI-powered tools can perform comprehensive literature reviews in a fraction of the time it would take a human researcher. By scanning thousands of publications, AI can identify under-researched areas, highlight inconsistencies in findings, and pinpoint topics that have not been adequately explored. For example, AI algorithms can map the frequency and distribution of certain keywords or concepts across publications, revealing topics that are either overrepresented or underrepresented in the literature.
While AI can efficiently analyze vast amounts of data, it is essential to maintain a human-in-the-loop approach. Human researchers are crucial in ensuring the correctness and relevance of the AI-generated insights. AI may identify a potential gap based on patterns in the data, but human expertise is necessary to evaluate whether the gap is genuinely significant and to provide the necessary clinical or scientific context. A human in the loop ensures that biases, misinterpretations, or irrelevant results are filtered out, improving the overall accuracy and validity of the findings.
- Trend Analysis
AI can track trends in research by analyzing the publication dates, authorship patterns, and citation networks of scientific papers. This analysis can reveal emerging areas of interest, shifts in research focus, and the lifecycle of topics. By understanding these trends, researchers can identify when a field is reaching saturation and where new questions are beginning to emerge.
- Sentiment Analysis
NLP techniques enable AI to perform sentiment analysis on research articles, identifying the tone and sentiment expressed in the literature. By analyzing the positive, negative, or neutral language used in studies, AI can detect areas of controversy, skepticism, or confidence within a field. This information can guide researchers toward topics that require further investigation or areas where there is a lack of consensus.
- Predictive Analytics
AI’s predictive capabilities can forecast future research trends based on historical data. By analyzing past and present research outputs, AI can predict which areas are likely to gain attention in the future and where potential research gaps may arise. This foresight allows researchers to position themselves at the forefront of emerging fields, contributing to innovative studies that address anticipated knowledge gaps.
AI in Suggesting New Research Opportunities
Beyond identifying existing research gaps, AI has the potential to suggest new research opportunities. By integrating data from multiple sources, AI can uncover connections and correlations that may not be immediately apparent, leading to the generation of novel hypotheses and research questions.
- Cross-Disciplinary Research
AI can facilitate cross-disciplinary research by identifying intersections between different fields of study. For example, by analyzing literature from both oncology and immunology, AI might identify a potential link between cancer treatment and immune response that has not been fully explored. These cross-disciplinary insights can lead to innovative research that bridges gaps between traditionally separate fields.
Read More: Predictive Analytics in Medical Research: The Role of AI
- Data-Driven Hypotheses
AI’s ability to analyze large datasets enables the generation of data-driven hypotheses. By examining patterns and correlations within clinical data, patient records, and genetic information, AI can suggest new avenues for research that are grounded in empirical evidence. These hypotheses can then be tested in clinical trials or experimental studies, potentially leading to breakthroughs in medical science.
- Real-World Data Integration
AI can integrate real-world data, such as electronic health records (EHRs), wearable device data, and social media activity, into the research process. By analyzing this data, AI can identify patterns and trends that may not be visible in traditional clinical studies. This real-world evidence can highlight gaps in current medical knowledge and suggest new research opportunities that are more aligned with the needs and experiences of patients.
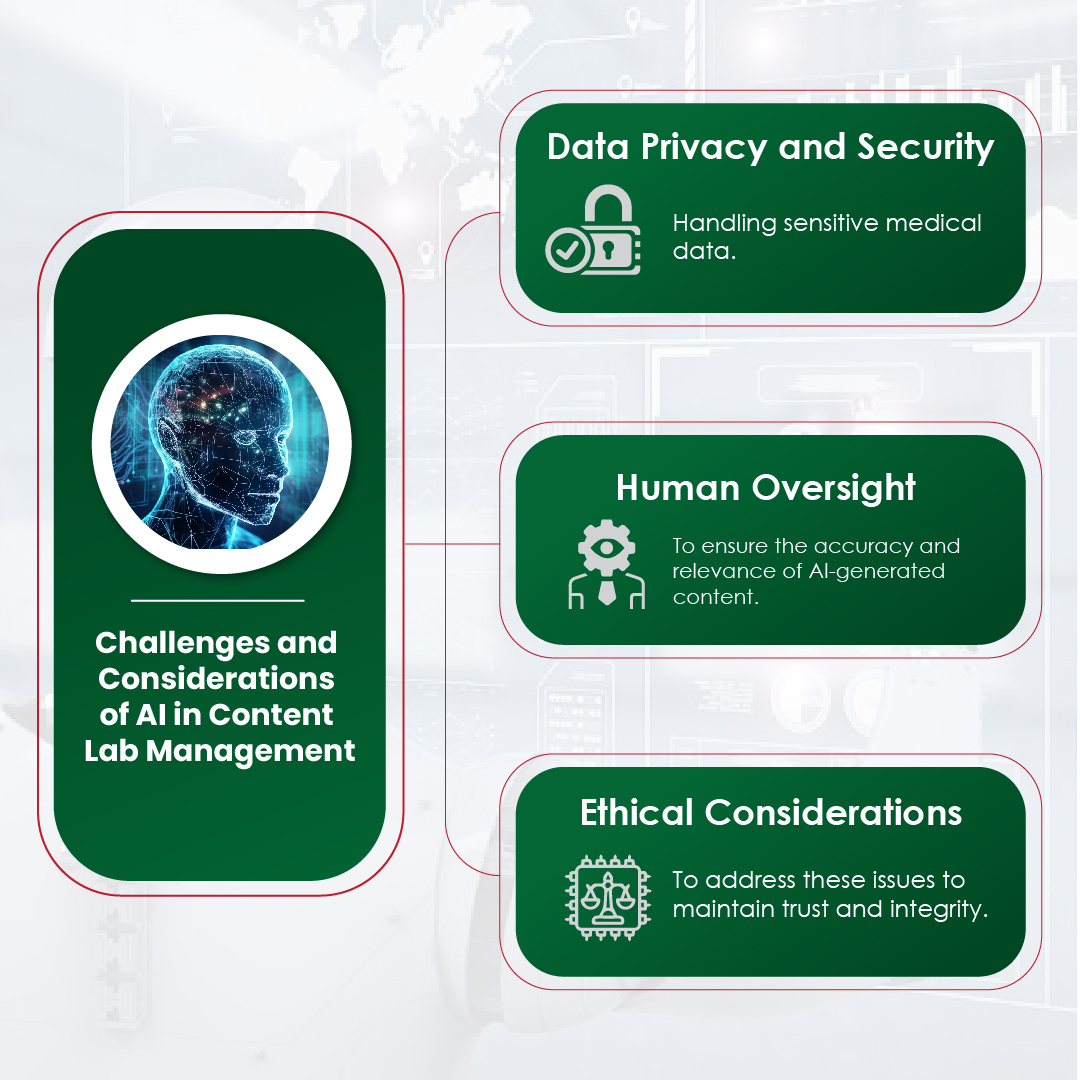
Challenges and Considerations
While AI offers significant advantages in identifying research gaps and opportunities, it is not without its challenges. The quality of AI-driven insights depends on the quality of the data it analyzes. Incomplete or biased datasets can lead to incorrect conclusions and missed opportunities. Therefore, it is crucial for researchers to ensure that the data fed into AI algorithms is comprehensive, diverse, and representative of the broader population.
AI algorithms may generate insights based on patterns in the data, but these insights require human interpretation and validation. Human researchers bring critical thinking, domain expertise, and the ability to assess the broader scientific context that AI lacks. Additionally, AI systems may sometimes generate false positives or overlook subtle nuances that are crucial in the interpretation of research gaps and opportunities.
Monitoring AI systems and ensuring proper checks and balances are in place is vital for the integrity of the research process. AI can suggest promising avenues of research, but human researchers must critically evaluate and refine these suggestions to ensure that they align with scientific goals and ethical standards.
AI is transforming the way researchers identify gaps in the medical literature and uncover new opportunities for investigation. By automating literature reviews, analyzing trends, and generating data-driven hypotheses, AI enables researchers to focus on the most promising areas of study and contribute to the advancement of medical science. However, the successful integration of AI into the research process requires careful consideration of data quality and a collaborative approach that leverages the strengths of both AI and human expertise.
As AI continues to evolve, its role in medical research will likely expand, offering even more sophisticated tools for identifying research gaps and suggesting new opportunities. For researchers and medical communication professionals, embracing AI’s potential is key to staying at the forefront of scientific discovery and innovation.