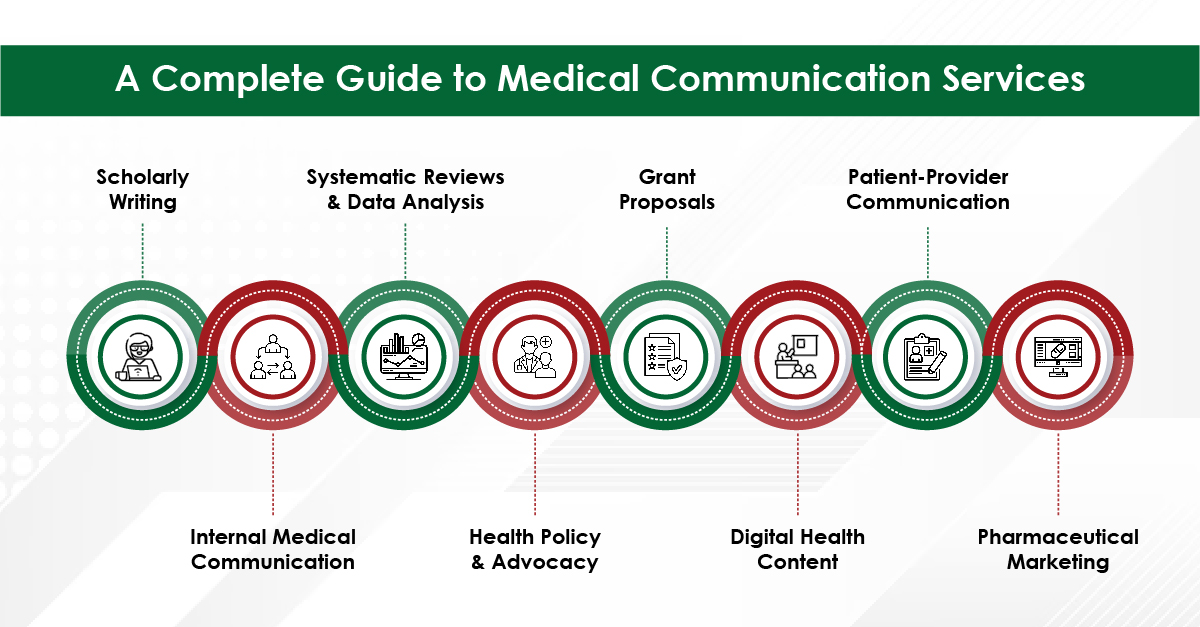
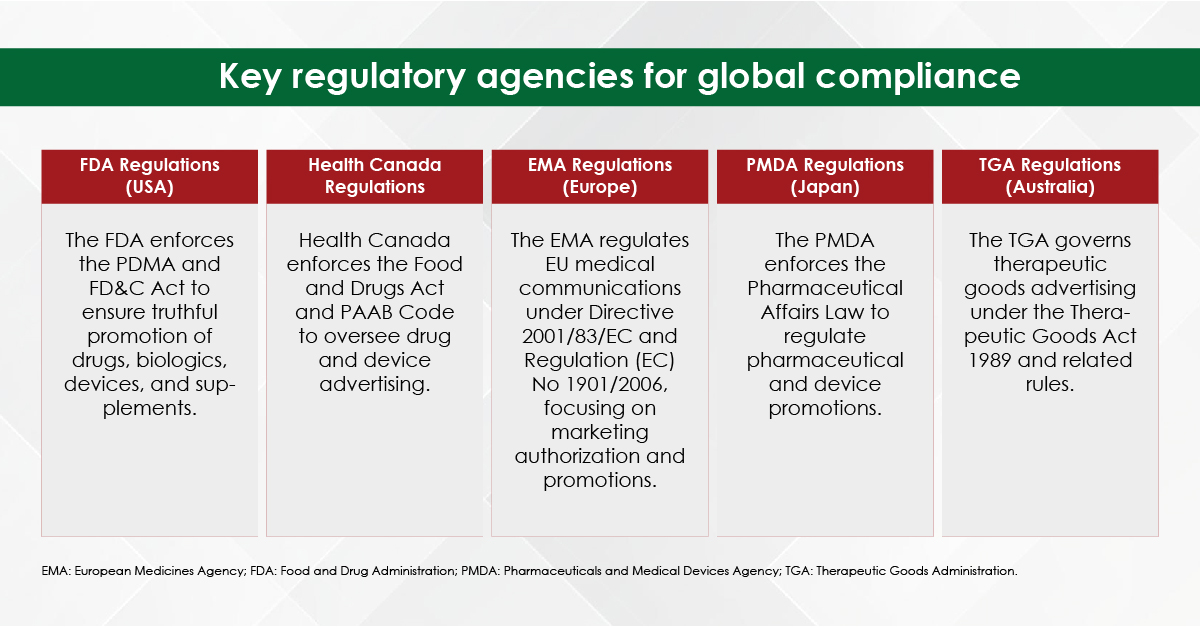
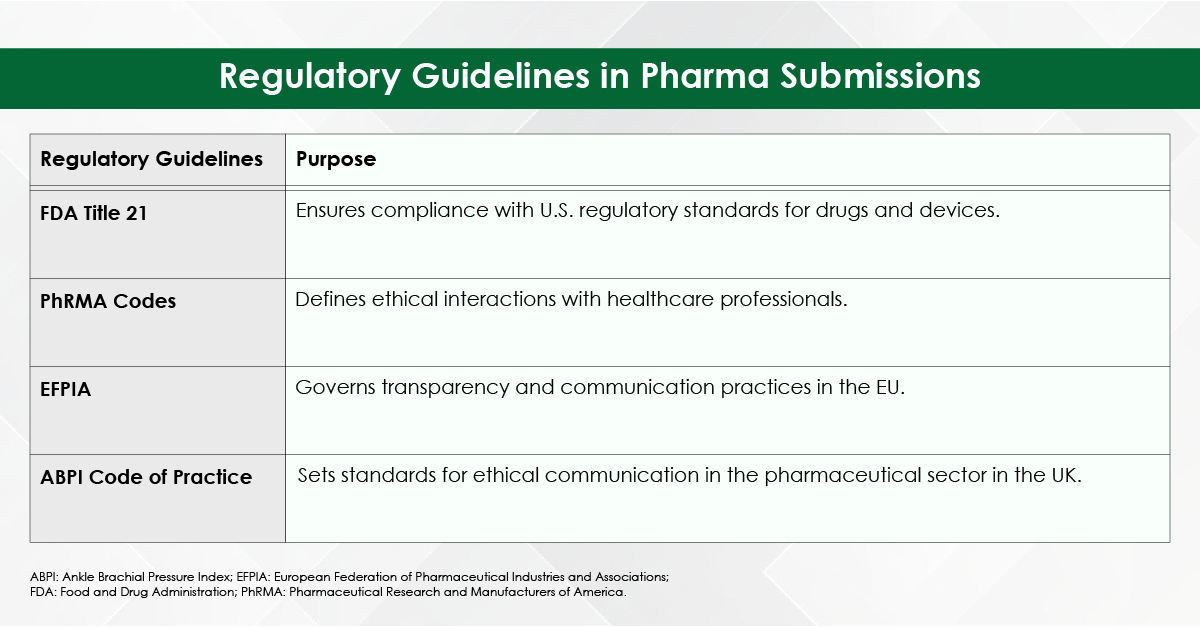
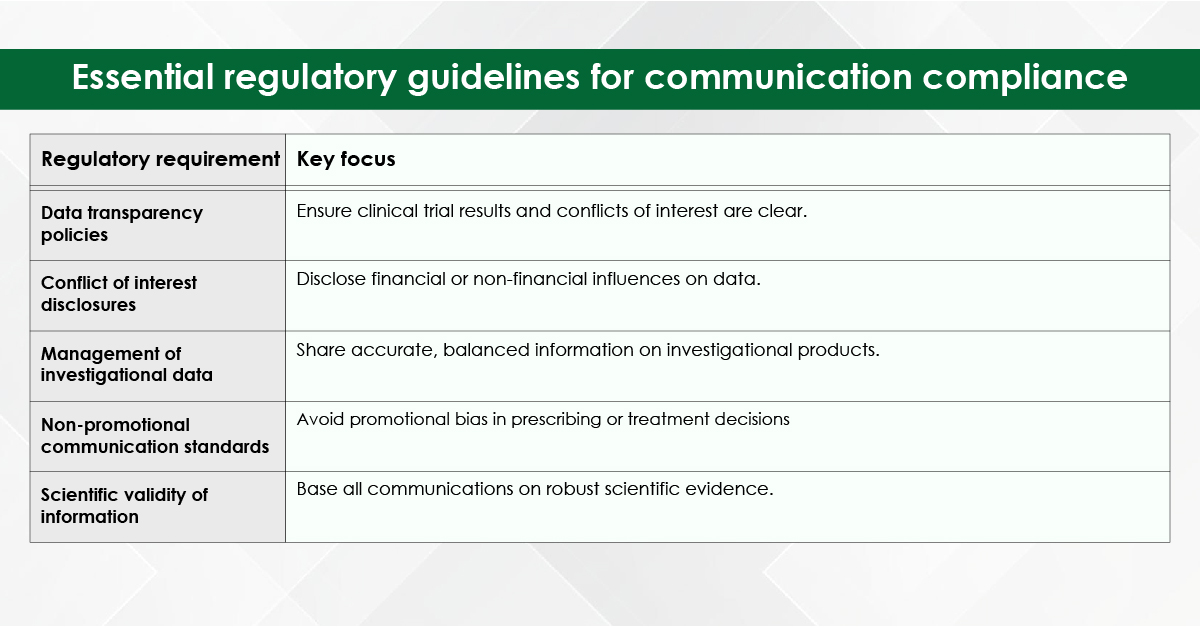
Medical writing demands utmost precision, as even a small error, such as a misplaced comma, can lead to confusion. While choosing the best medical writing agency for your needs, it is important to keep in mind that they should be proficient in medical terminologies and possess the expertise to create and process various types of documents—whether for scientific audiences, the general public, or regulatory agencies—each requiring unique writing and presentation styles.
Step-by-Step Guide to Choosing the Right Medical Writing Agency
The following step-by-step guide will help you identify the ideal medical writing partner. By following these expert tips, you can ensure that your project meets the highest standards of quality, precision, and professionalism:

- Understand Your Audience
A reliable content agency takes the time to analyze your target audience’s demographics, preferences, and needs. This ensures that the medical content is accurate, engaging, and tailored to resonate effectively with its intended readers.
- Experienced and Specialized Writers
Leading content agencies have a team of highly skilled writers with expertise in various healthcare domains. These professionals are well-versed in crafting content that meets the highest regulatory standards, ensuring accuracy, compliance, and credibility in every project.
- Commitment to Timely Delivery
Time is crucial in the healthcare and pharmaceutical industries. A reliable agency must prioritize deadlines, consistently deliver high-quality content on time, and adapt seamlessly to sudden increases in content demands to keep project timelines on track.
- Scalability and Flexibility
The right partner offers scalable solutions to meet fluctuating content demands. Whether you’re handling a small project or a large-scale initiative, they ensure seamless production without requiring you to invest in additional resources or personnel.
- Technology and Innovation
Agencies leveraging advanced tools like AI-driven writing platforms, content management systems, or data analytics to enhance efficiency and accuracy.
- Global Reach
For multinational projects, it’s essential to partner with an agency that excels in localization and understands cultural nuances. This ensures your content resonates with diverse audiences while maintaining accuracy and relevance. An experienced agency will adapt language, tone, and context to meet the specific needs of each target market.
Why choose a medical writing agency
Partnering with a medical writing agency offers specialized expertise, streamlined workflows, and high-quality, compliant content tailored to your needs, freeing up valuable time to drive business growth.
A medical writing agency plays a pivotal role in content creation, developing everything from website content and whitepapers to social media posts, all customized to meet your specific goals. They manage the entire content creation process—from initial concept planning to the final draft—ensuring every piece meets the highest standards of accuracy and professionalism. This end-to-end approach allows you to focus on strategic business priorities while leaving the details to the experts.
Five Steps for Choosing the Right Content Writing Agency
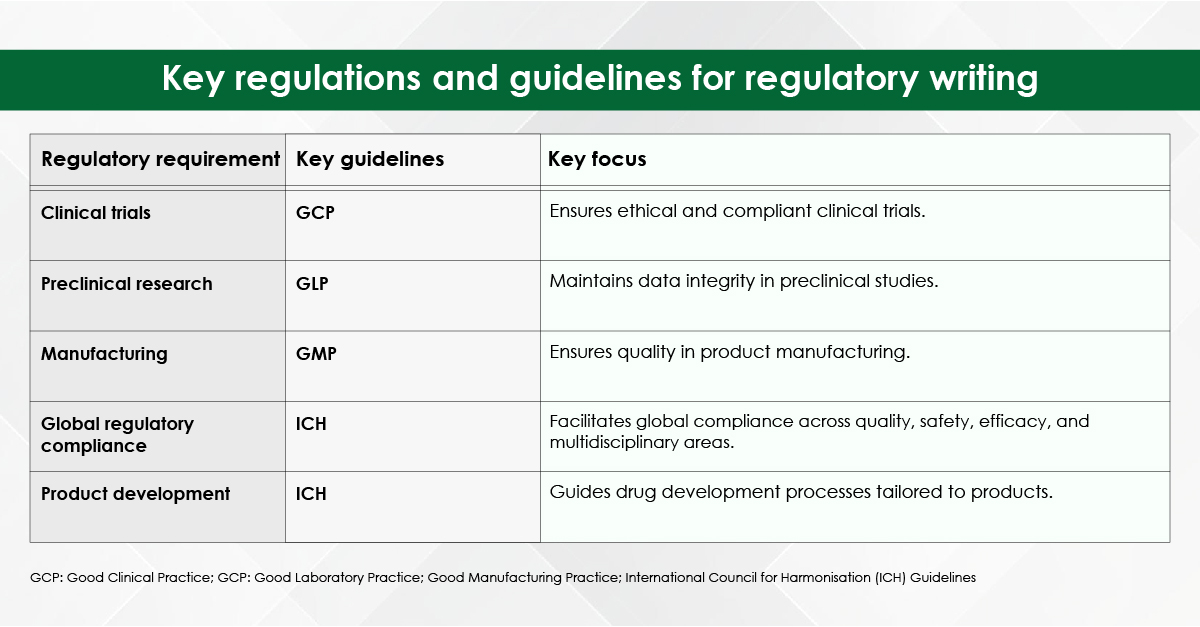
Finding the right medical agency that fits your business will take a bit of elbow grease. It’s challenging to locate an agency proficient in various types of medical publishing. These include journal publishing, report writing, scientific communication, data monitoring, omnichannel communications, CSR clinical studies, clinical trial studies, treatment planning analysis reports, omnichannel promotional/non-promotional communication, and content strategy planning.
- Determine What You Need
The first step is to determine what content you need from your agency. Understanding your needs will ensure you choose an agency as when you need it, and how you want it done.
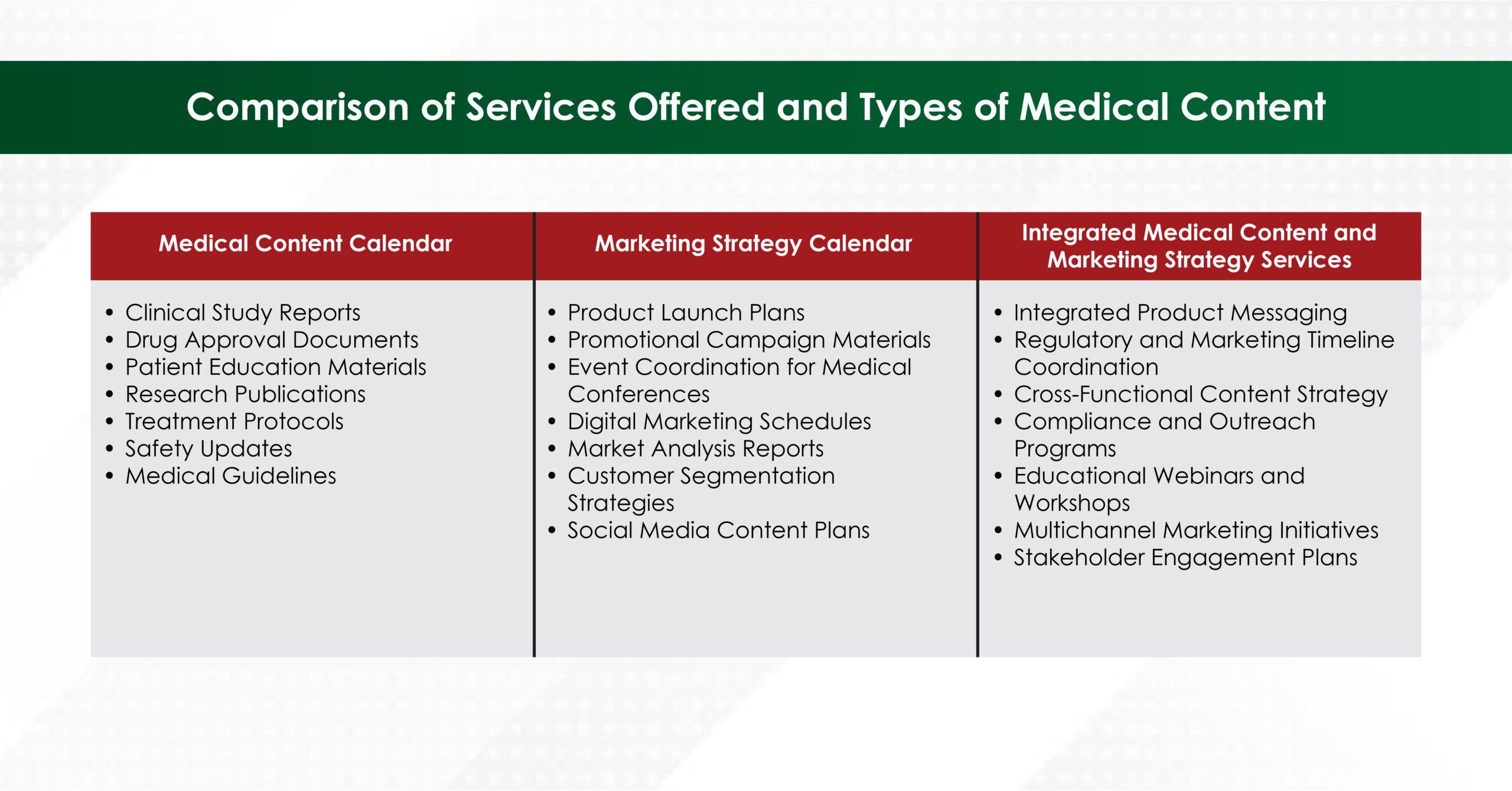
- What If I Don’t Know What I Need?
If you’re unsure about your content requirements, consider choosing an agency that offers separate services for medical content calendars and marketing strategy calendars. Such agencies can create a well-defined content plan tailored to help you achieve your goals.
- Evaluate Content Agency Expertise
When selecting a top-tier agency for medical writing in clinical research, it’s essential to ensure they operate across all major therapeutic areas and deliver tailored solutions for every stage of drug development, from early clinical phases to post-marketing activities. Look for specialization in key areas such as:
- Early-phase Clinical Trials
Expertly documenting trial protocols, results, and analysis for initial drug development stages.
- Post-marketing Content
Generating real-world evidence and safety updates to support ongoing product lifecycle management.
- Scientific Communications
Creating impactful presentations, abstracts, and manuscripts for conferences and peer-reviewed journals.
- Marketing Authorization Applications
Crafting regulatory-compliant dossiers for product approval and market entry.
- Assess Collaboration and Communication
A reliable content agency dealing in healthcare ensures close collaboration by providing regular updates and maintaining clear communication throughout the project.
Look for an agency that assigns a dedicated contact for updates, leverages collaborative tools like shared calendars and review platforms, and ensures transparency in workflows, timelines, and deliverables for efficient and seamless project management.
- Review Credentials and Past Work
An agency’s track record and portfolio provide valuable insights into their expertise and reliability. Review their case studies, client testimonials, sample documents from similar projects, and certifications or affiliations with regulatory and medical writing associations to assess their credibility and capability.
Final Thoughts
Partnering with the right medical writing agency is vital to achieving your business goals. By carefully assessing your needs, evaluating the agency’s expertise, and ensuring seamless collaboration, you can choose a partner who delivers exceptional content, adheres to international standards, and aligns with your broader objectives. Consider exploring the comprehensive medical writing services offered by Turacoz, where expertise meets precision to help you achieve outstanding results.
Why choose Turacoz for medical writing?
At Turacoz, we offer a comprehensive range of medical writing services tailored to meet diverse needs across the healthcare and pharmaceutical industries. Our Regulatory Writing Services include medical writing in clinical research & trial reports, investigator brochures, and marketing authorization applications, ensuring compliance with international standards. In Scientific Communications, we specialize in research publications, conference abstracts, posters, and literature reviews. Our Medical Marketing Content covers product launch materials, promotional campaigns, and digital marketing strategies. We also focus on Patient Education and Outreach, providing disease awareness campaigns, treatment guides, and educational videos. Additionally, our expertise extends to Real-World Evidence (RWE) and HEOR Writing, including cost-effectiveness analyses, market access dossiers, and observational study reports. For Health and Wellness Content, we create blogs, newsletters, and lifestyle articles. Our Training and Educational Materials cater to e-learning modules, CME content, and webinar presentations. Furthermore, we offer Conference and Event Support, covering pre-event briefs, on-site coverage, and post-event summaries. With Turacoz, you gain a trusted partner capable of delivering high-quality, precise, and impactful content across all facets of medical writing.